CARD9 mediates dectin-2-induced IkappaBalpha kinase ubiquitination leading to activation of NF-kappaB in response to stimulation by the hyphal form of Candida albicans
- PMID: 20538615
- PMCID: PMC2923990
- DOI: 10.1074/jbc.M110.131300
CARD9 mediates dectin-2-induced IkappaBalpha kinase ubiquitination leading to activation of NF-kappaB in response to stimulation by the hyphal form of Candida albicans
Abstract
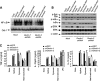
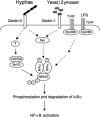
The scaffold protein CARD9 plays an essential role in anti-fungus immunity and is implicated in mediating Dectin-1/Syk-induced NF-kappaB activation in response to Candida albicans infection. However, the molecular mechanism by which CARD9 mediates C. albicans-induced NF-kappaB activation is not fully characterized. Here we demonstrate that CARD9 is involved in mediating NF-kappaB activation induced by the hyphal form of C. albicans hyphae (Hyphae) but not by its heat-inactivated unicellular form. Our data show that inhibiting Dectin-2 expression selectively blocked Hyphae-induced NF-kappaB, whereas inhibiting Dectin-1 mainly suppressed zymosan-induced NF-kappaB, indicating that Hyphae-induced NF-kappaB activation is mainly through Dectin-2 and not Dectin-1. Consistently, we find that the hyphae stimulation induces CARD9 association with Bcl10, an adaptor protein that functions downstream of CARD9 and is also involved in C. albicans-induced NF-kappaB activation. This association is dependent on Dectin-2 but not Dectin-1 following the hyphae stimulation. Finally, we find that although both CARD9 and Syk are required for Hyphae-induced NF-kappaB activation, they regulate different signaling events in which CARD9 mediates IkappaBalpha kinase ubiquitination, whereas Syk regulates IkappaBalpha kinase phosphorylation. Together, our data demonstrated that CARD9 is selectively involved in Dectin-2-induced NF-kappaB activation in response to C. albicans hyphae challenging.
Figures


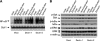


References
-
- Netea M. G., Brown G. D., Kullberg B. J., Gow N. A. (2008) Nat. Rev. Microbiol. 6, 67–78 - PubMed
-
- Robinson M. J., Sancho D., Slack E. C., LeibundGut-Landmann S., Reis e Sousa C. (2006) Nat. Immunol. 7, 1258–1265 - PubMed
-
- Willment J. A., Brown G. D. (2008) Trends Microbiol. 16, 27–32 - PubMed
-
- Hayden M. S., Ghosh S. (2008) Cell 132, 344–362 - PubMed
-
- Karin M., Ben-Neriah Y. (2000) Annu. Rev. Immunol. 18, 621–663 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

