MALDI-TOF/MS analysis of archaebacterial lipids in lyophilized membranes dry-mixed with 9-aminoacridine
- PMID: 20538644
- PMCID: PMC2918464
- DOI: 10.1194/jlr.D007328
MALDI-TOF/MS analysis of archaebacterial lipids in lyophilized membranes dry-mixed with 9-aminoacridine
Abstract
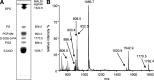
A method of direct lipid analysis by MALDI mass spectrometry in intact membranes, without prior extraction/separation steps, is described. The purple membrane isolated from the extremely halophilic archaeon Halobacterium salinarum was selected as model membrane. Lyophilized purple membrane were grinded with 9-aminoacridine (9-AA) as dry matrix, and the powder mixture was crushed in a mechanical die press to form a thin pellet. Small pieces of the pellet were then attached to the MALDI target and directly analyzed. In parallel, individual archaebacterial phospholipids and glycolipids, together with the total lipid extract of the purple membrane, were analyzed by MALDI-TOF/MS using 9-AA as the matrix in solution. Results show that 9-AA represents a suitable matrix for the conventional MALDI-TOF/MS analysis of lipid extracts from archaeal microorganisms, as well as for fast and reliable direct dry lipid analysis of lyophilized archaebacterial membranes. This method might be of general application, offering the advantage of quickly gaining information about lipid components without disrupting or altering the membrane matrix.
Figures





References
-
- Han X., Gross R. W. 2003. Global analyses of cellular lipidomes directly from crude extracts of biological samples by ESI mass spectrometry: a bridge to lipidomics. J. Lipid Res. 44: 1071–1079. - PubMed
-
- Fahy E., Subramaniam S., Brown H. A., Glass C. K., Jr. Merrill A. H., Murphy R. C., Raetz C. R. H., Russell D. W., Seyama Y., Shaw W., et al. 2005. A comprehensive classification system for lipids. J. Lipid Res. 46: 839–861. - PubMed
-
- Kates M. 1993. The Biochemistry of Archaea (Archaebacteria). Kates M., Kushner D. J., Matheson A. T., Elsevier Science Publishers, Amsterdam.
-
- Kamekura M., Kates M. 1988. Lipids of Halophilic Archaebacteria. Halophilic bacteria. Rodriguez Valera F., editor CRC press, Boca Raton, Florida, FL: Vol. II, pp. 25–54.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

