Alterations of L-type calcium current and cardiac function in CaMKII{delta} knockout mice
- PMID: 20538682
- PMCID: PMC2923749
- DOI: 10.1161/CIRCRESAHA.110.222562
Alterations of L-type calcium current and cardiac function in CaMKII{delta} knockout mice
Abstract
Rationale: Recent studies have highlighted important roles of CaMKII in regulating Ca(2+) handling and excitation-contraction coupling. However, the cardiac effect of chronic CaMKII inhibition has not been well understood.
Objective: We have tested the alterations of L-type calcium current (I(Ca)) and cardiac function in CaMKIIdelta knockout (KO) mouse left ventricle (LV).
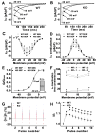
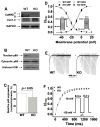
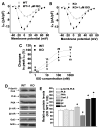
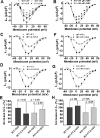
Methods and results: We used the patch-clamp method to record I(Ca) in ventricular myocytes and found that in KO LV, basal I(Ca) was significantly increased without changing the transmural gradient of I(Ca) distribution. Substitution of Ba(2+) for Ca(2+) showed similar increase in I(Ba). There was no change in the voltage dependence of I(Ca) activation and inactivation. I(Ca) recovery from inactivation, however, was significantly slowed. In KO LV, the Ca(2+)-dependent I(Ca) facilitation (CDF) and I(Ca) response to isoproterenol (ISO) were significantly reduced. However, ISO response was reversed by beta2-adrenergic receptor (AR) inhibition. Western blots showed a decrease in beta1-AR and an increase in Ca(v)1.2, beta2-AR, and Galphai3 protein levels. Ca(2+) transient and sarcomere shortening in KO myocytes were unchanged at 1-Hz but reduced at 3-Hz stimulation. Echocardiography in conscious mice revealed an increased basal contractility in KO mice. However, cardiac reserve to work load and beta-adrenergic stimulation was reduced. Surprisingly, KO mice showed a reduced heart rate in response to work load or beta-adrenergic stimulation.
Conclusions: Our results implicate physiological CaMKII activity in maintaining normal I(Ca), Ca(2+) handling, excitation-contraction coupling, and the in vivo heart function in response to cardiac stress.
Conflict of interest statement
There is no conflict to disclose.
Figures

References
-
- Tobimatsu T, Fujisawa H. Tissue-specific expression of four types of rat calmodulin-dependent protein kinase II mRNAs. J Biol Chem. 1989;264:17907–17912. - PubMed
-
- Baltas LG, Karczewski P, Krause EG. The cardiac sarcoplasmic reticulum phospholamban kinase is a distinct delta-CaM kinase isozyme. FEBS Lett. 1995;373:71–75. - PubMed
-
- Singer HA, Benscoter HA, Schworer CM. Novel Ca2+/calmodulin-dependent protein kinase II gamma-subunit variants expressed in vascular smooth muscle, brain, and cardiomyocytes. J Biol Chem. 1997;272:9393–9400. - PubMed
-
- Hoch B, Haase H, Schulze W, Hagemann D, Morano I, Krause EG, Karczewski P. Differentiation-dependent expression of cardiac delta-CaMKII isoforms. J Cell Biochem. 1998;68:259–268. - PubMed
-
- Anderson ME. Calmodulin kinase and L-type calcium channels; a recipe for arrhythmias? Trends Cardiovasc Med. 2004;14:152–161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

