The roles of somatostatin-expressing (GIN) and fast-spiking inhibitory interneurons in UP-DOWN states of mouse neocortex
- PMID: 20538767
- PMCID: PMC2934925
- DOI: 10.1152/jn.00206.2010
The roles of somatostatin-expressing (GIN) and fast-spiking inhibitory interneurons in UP-DOWN states of mouse neocortex
Abstract
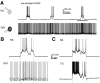
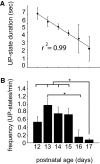
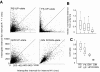
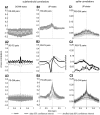
The neocortex contains multiple types of inhibitory neurons whose properties suggest they may play different roles within the cortical circuit. By recording from three cell types during two distinct network states (UP and DOWN states) in vitro, we were able to quantify differences in firing characteristics between these cells during different network regimes. We recorded from regular-spiking (RS) excitatory cells and two types of inhibitory neurons, the fast-spiking (FS) neurons and GFP- (and somatostatin-) expressing inhibitory neurons (GIN), in layer 2/3 of slices from mouse somatosensory neocortex. Comparisons of firing characteristics between these cells during UP- and DOWN-states showed several patterns. First, of these cell types, only GIN cells fired persistently during DOWN-states, whereas all three cell types fired readily during UP-states. Second, the onset of firing and distribution of action potentials throughout UP-states differed by cell type, showing that FS cell UP-state firing occurred preferentially near the beginning of the UP-state, whereas the firing of RS cells was slower to develop at the start of the UP-state, and GIN cell firing was sustained throughout the duration of the UP-state. Finally, membrane potential and spike correlations between heterogeneous cell types were more pronounced during UP-states and, in the case of RS synapses onto GIN cells, varied throughout the UP-state. These results suggest that there is a division of labor between FS and GIN cells as the UP-state progresses and suggest that GIN cells could be important in the termination of UP-states.
Figures



Similar articles
-
Selective, state-dependent activation of somatostatin-expressing inhibitory interneurons in mouse neocortex.J Neurophysiol. 2008 Nov;100(5):2640-52. doi: 10.1152/jn.90691.2008. Epub 2008 Sep 17. J Neurophysiol. 2008. PMID: 18799598 Free PMC article.
-
Differential Excitation of Distally versus Proximally Targeting Cortical Interneurons by Unitary Thalamocortical Bursts.J Neurosci. 2016 Jun 29;36(26):6906-16. doi: 10.1523/JNEUROSCI.0739-16.2016. J Neurosci. 2016. PMID: 27358449 Free PMC article.
-
Laminar specificity of functional input to distinct types of inhibitory cortical neurons.J Neurosci. 2009 Jan 7;29(1):70-85. doi: 10.1523/JNEUROSCI.4104-08.2009. J Neurosci. 2009. PMID: 19129386 Free PMC article.
-
Somatostatin-Expressing Inhibitory Interneurons in Cortical Circuits.Front Neural Circuits. 2016 Sep 29;10:76. doi: 10.3389/fncir.2016.00076. eCollection 2016. Front Neural Circuits. 2016. PMID: 27746722 Free PMC article. Review.
-
Somatostatin-expressing neurons in cortical networks.Nat Rev Neurosci. 2016 Jul;17(7):401-9. doi: 10.1038/nrn.2016.53. Epub 2016 May 26. Nat Rev Neurosci. 2016. PMID: 27225074 Free PMC article. Review.
Cited by
-
In vivo measurement of cell-type-specific synaptic connectivity and synaptic transmission in layer 2/3 mouse barrel cortex.Neuron. 2015 Jan 7;85(1):68-75. doi: 10.1016/j.neuron.2014.11.025. Epub 2014 Dec 24. Neuron. 2015. PMID: 25543458 Free PMC article.
-
A distinct class of slow (~0.2-2 Hz) intrinsically bursting layer 5 pyramidal neurons determines UP/DOWN state dynamics in the neocortex.J Neurosci. 2015 Apr 8;35(14):5442-58. doi: 10.1523/JNEUROSCI.3603-14.2015. J Neurosci. 2015. PMID: 25855163 Free PMC article.
-
A blanket of inhibition: functional inferences from dense inhibitory connectivity.Curr Opin Neurobiol. 2014 Jun;26:96-102. doi: 10.1016/j.conb.2013.12.015. Epub 2014 Jan 15. Curr Opin Neurobiol. 2014. PMID: 24440415 Free PMC article.
-
EEG as a translational biomarker and outcome measure in fragile X syndrome.Transl Psychiatry. 2022 Jan 24;12(1):34. doi: 10.1038/s41398-022-01796-2. Transl Psychiatry. 2022. PMID: 35075104 Free PMC article. Review.
-
Hippocampal GABAergic Inhibitory Interneurons.Physiol Rev. 2017 Oct 1;97(4):1619-1747. doi: 10.1152/physrev.00007.2017. Physiol Rev. 2017. PMID: 28954853 Free PMC article. Review.
References
-
- Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience 41: 365–379, 1991 - PubMed
-
- Bacci A, Huguenard JR. Enhancement of spike-timing precision by autaptic transmission in neocortical inhibitory interneurons. Neuron 49: 119–130, 2006 - PubMed
-
- Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci 8: 451–465, 2007 - PubMed
-
- Beierlein M, Gibson JR, Connors BW. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J Neurophysiol 90: 2987–3000, 2003 - PubMed
-
- Compte A, Sanchez-Vives MV, McCormick DA, Wang XJ. Cellular and network mechanisms of slow oscillatory activity (<1 Hz) and wave propagations in a cortical network model. J Neurophysiol 89: 2707–2725, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

