Effect of vascular endothelial growth factor and its receptor KDR on the transendothelial migration and local trafficking of human T cells in vitro and in vivo
- PMID: 20538805
- PMCID: PMC3173992
- DOI: 10.1182/blood-2009-11-252460
Effect of vascular endothelial growth factor and its receptor KDR on the transendothelial migration and local trafficking of human T cells in vitro and in vivo
Abstract
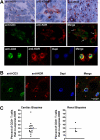
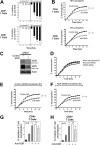
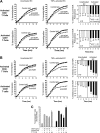
In these studies, we find that the vascular endothelial growth factor (VEGF) receptor KDR is expressed on subsets of mitogen-activated CD4(+) and CD8(+) T cells in vitro. We also found that KDR colocalizes with CD3 on mitogen-activated T cells in vitro and on infiltrates within rejecting human allografts in vivo. To evaluate whether VEGF and KDR mediate lymphocyte migration across endothelial cells (ECs), we used an in vitro live-time transmigration model and observed that both anti-VEGF and anti-KDR antibodies inhibit the transmigration of both CD4(+) and CD8(+) T cells across tumor necrosis factor α (TNFα)-activated, but not unactivated ECs. In addition, we found that interactions among CD4(+) or CD8(+) T cells and TNFα-activated ECs result in the induction of KDR on each T cell subset, and that KDR-expressing lymphocytes preferentially transmigrate across TNFα-activated ECs. Finally, using a humanized severe combined immunodeficient mouse model of lymphocyte trafficking, we found that KDR-expressing lymphocytes migrate into human skin in vivo, and that migration is reduced in mice treated with a blocking anti-VEGF antibody. These observations demonstrate that induced expression of KDR on subsets of T cells, and locally expressed VEGF, facilitate EC-dependent lymphocyte chemotaxis, and thus, the localization of T cells at sites of inflammation.
Figures

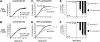

Similar articles
-
FMS-like tyrosine kinase 1 (FLT1) is a key regulator of fetoplacental endothelial cell migration and angiogenesis.Placenta. 2018 Oct;70:7-14. doi: 10.1016/j.placenta.2018.08.004. Epub 2018 Aug 20. Placenta. 2018. PMID: 30316329 Free PMC article.
-
Inhibition of both the autocrine and the paracrine growth of human leukemia with a fully human antibody directed against vascular endothelial growth factor receptor 2.Leuk Lymphoma. 2004 Sep;45(9):1887-97. doi: 10.1080/10428190410001712225. Leuk Lymphoma. 2004. PMID: 15223651
-
Inhibition of human leukemia in an animal model with human antibodies directed against vascular endothelial growth factor receptor 2. Correlation between antibody affinity and biological activity.Leukemia. 2003 Mar;17(3):604-11. doi: 10.1038/sj.leu.2402831. Leukemia. 2003. PMID: 12646950
-
Stimulatory effect of Cinnamomum cassia and cinnamic acid on angiogenesis through up-regulation of VEGF and Flk-1/KDR expression.Int Immunopharmacol. 2009 Jul;9(7-8):959-67. doi: 10.1016/j.intimp.2009.04.001. Epub 2009 Apr 6. Int Immunopharmacol. 2009. PMID: 19356742
-
Towards Targeting Endothelial Rap1B to Overcome Vascular Immunosuppression in Cancer.Int J Mol Sci. 2024 Sep 12;25(18):9853. doi: 10.3390/ijms25189853. Int J Mol Sci. 2024. PMID: 39337337 Free PMC article. Review.
Cited by
-
Targeting the intragraft microenvironment and the development of chronic allograft rejection.Hum Immunol. 2012 Dec;73(12):1261-8. doi: 10.1016/j.humimm.2012.07.334. Epub 2012 Aug 3. Hum Immunol. 2012. PMID: 22863981 Free PMC article. Review.
-
Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein.Proc Natl Acad Sci U S A. 2013 Aug 13;110(33):13588-93. doi: 10.1073/pnas.1308673110. Epub 2013 Jul 29. Proc Natl Acad Sci U S A. 2013. PMID: 23898208 Free PMC article.
-
Inhibition of Vascular Endothelial Growth Factor Receptors 1 and 2 Attenuates Natural Killer Cell and Innate Immune Responses in an Experimental Model for Obliterative Bronchiolitis.Am J Pathol. 2022 Feb;192(2):254-269. doi: 10.1016/j.ajpath.2021.10.018. Epub 2021 Nov 11. Am J Pathol. 2022. PMID: 34774518 Free PMC article.
-
Biological and Clinical Implications of the Vascular Endothelial Growth Factor Coreceptor Neuropilin-1 in Human Immunodeficiency Virus.Open Forum Infect Dis. 2023 Sep 8;10(10):ofad467. doi: 10.1093/ofid/ofad467. eCollection 2023 Oct. Open Forum Infect Dis. 2023. PMID: 37869406 Free PMC article.
-
Improving the Efficiency of Vγ9Vδ2 T-Cell Immunotherapy in Cancer.Front Immunol. 2018 Apr 19;9:800. doi: 10.3389/fimmu.2018.00800. eCollection 2018. Front Immunol. 2018. PMID: 29725332 Free PMC article. Review.
References
-
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246(4935):1306–1309. - PubMed
-
- Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219(4587):983–985. - PubMed
-
- Reinders ME, Rabelink TJ, Briscoe DM. Angiogenesis and endothelial cell repair in renal disease and allograft rejection. J Am Soc Nephrol. 2006;17(4):932–942. - PubMed
-
- Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med. 1999;5(12):1359–1364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

