Virus-induced Ca2+ influx extends survival of west nile virus-infected cells
- PMID: 20538858
- PMCID: PMC2918993
- DOI: 10.1128/JVI.00144-10
Virus-induced Ca2+ influx extends survival of west nile virus-infected cells
Abstract
West Nile virus (WNV) infection leads to rapid and sustained Ca(2+) influx. This influx was observed with different strains of WNV and in different types of cells. Entry during virion endocytosis as well as through calcium channels contributed to the Ca(2+) influx observed in WNV-infected cells. Ca(2+) influx was not detected after infection with vesicular stomatitis virus (VSV) and occurred only through endocytosis in Sindbis virus-infected cells. Caspase 3 cleavage and activation of several kinases, including focal adhesion kinase (FAK), mitogen-activated extracellular signal-regulated protein kinase (ERK1/2), and protein-serine kinase B alpha (Akt), at early times after WNV infection were shown to be dependent on Ca(2+) influx. Although the activation of these kinases was sustained in virus-infected cells throughout infection, UV-inactivated WNV induced only a transient activation of FAK and ERK1/2 at early times after infection. The Ca(2+)-dependent FAK activation observed in WNV-infected cells was not mediated by alphavbeta3 integrins. Reduction of Ca(2+) influx at early times of infection by various treatments decreased the viral yield and delayed both the early transient caspase 3 cleavage and the activation of FAK, Akt, and ERK signaling. The results indicate that Ca(2+) influx is required for early infection events needed for efficient viral replication, possibly for virus-induced rearrangement of the endoplasmic reticulum (ER) membrane. Increased caspase 3 cleavage at both early (transient) and late times of infection correlated with decreased activation of the FAK and ERK1/2 pathways, indicating a role for these kinases in extending the survival of flavivirus-infected cells.
Figures


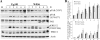

References
-
- Achison, M., C. M. Elton, P. G. Hargreaves, C. G. Knight, M. J. Barnes, and R. W. Farndale. 2001. Integrin-independent tyrosine phosphorylation of p125(fak) in human platelets stimulated by collagen. J. Biol. Chem. 276:3167-3174. - PubMed
-
- Alessandro, R., L. Masiero, K. Lapidos, J. Spoonster, and E. C. Kohn. 1998. Endothelial cell spreading on type IV collagen and spreading-induced FAK phosphorylation is regulated by Ca2+ influx. Biochem. Biophys. Res. Commun. 248:635-640. - PubMed
-
- Beasley, D. W., L. Li, M. T. Suderman, and A. D. Barrett. 2002. Mouse neuroinvasive phenotype of West Nile virus strains varies depending upon virus genotype. Virology 296:17-23. - PubMed
-
- Berridge, M. J., M. D. Bootman, and H. L. Roderick. 2003. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell. Biol. 4:517-529. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

