Mutant HSPB8 causes motor neuron-specific neurite degeneration
- PMID: 20538880
- PMCID: PMC2908473
- DOI: 10.1093/hmg/ddq234
Mutant HSPB8 causes motor neuron-specific neurite degeneration
Abstract
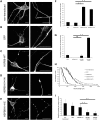
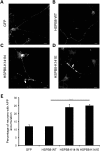
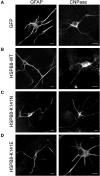
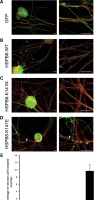
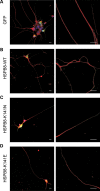
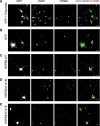
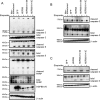
Missense mutations (K141N and K141E) in the alpha-crystallin domain of the small heat shock protein HSPB8 (HSP22) cause distal hereditary motor neuropathy (distal HMN) or Charcot-Marie-Tooth neuropathy type 2L (CMT2L). The mechanism through which mutant HSPB8 leads to a specific motor neuron disease phenotype is currently unknown. To address this question, we compared the effect of mutant HSPB8 in primary neuronal and glial cell cultures. In motor neurons, expression of both HSPB8 K141N and K141E mutations clearly resulted in neurite degeneration, as manifested by a reduction in number of neurites per cell, as well as in a reduction in average length of the neurites. Furthermore, expression of the K141E (and to a lesser extent, K141N) mutation also induced spheroids in the neurites. We did not detect any signs of apoptosis in motor neurons, showing that mutant HSPB8 resulted in neurite degeneration without inducing neuronal death. While overt in motor neurons, these phenotypes were only very mildly present in sensory neurons and completely absent in cortical neurons. Also glial cells did not show an altered phenotype upon expression of mutant HSPB8. These findings show that despite the ubiquitous presence of HSPB8, only motor neurons appear to be affected by the K141N and K141E mutations which explain the predominant motor neuron phenotype in distal HMN and CMT2L.
Figures
Similar articles
-
Phenotype of cardiomyopathy in cardiac-specific heat shock protein B8 K141N transgenic mouse.J Biol Chem. 2013 Mar 29;288(13):8910-21. doi: 10.1074/jbc.M112.368324. Epub 2013 Feb 6. J Biol Chem. 2013. PMID: 23389032 Free PMC article.
-
A knock-in/knock-out mouse model of HSPB8-associated distal hereditary motor neuropathy and myopathy reveals toxic gain-of-function of mutant Hspb8.Acta Neuropathol. 2018 Jan;135(1):131-148. doi: 10.1007/s00401-017-1756-0. Epub 2017 Aug 5. Acta Neuropathol. 2018. PMID: 28780615 Free PMC article.
-
Autophagy induction by piplartine ameliorates axonal degeneration caused by mutant HSPB1 and HSPB8 in Charcot-Marie-Tooth type 2 neuropathies.Autophagy. 2025 May;21(5):1116-1143. doi: 10.1080/15548627.2024.2439649. Epub 2024 Dec 27. Autophagy. 2025. PMID: 39698979 Free PMC article.
-
The Spectrum of Small Heat Shock Protein B8 (HSPB8)-Associated Neuromuscular Disorders.Int J Mol Sci. 2025 Mar 23;26(7):2905. doi: 10.3390/ijms26072905. Int J Mol Sci. 2025. PMID: 40243504 Free PMC article. Review.
-
Structure, properties, and functions of the human small heat-shock protein HSP22 (HspB8, H11, E2IG1): a critical review.J Neurosci Res. 2008 Feb 1;86(2):264-9. doi: 10.1002/jnr.21441. J Neurosci Res. 2008. PMID: 17722063 Review.
Cited by
-
De Novo and Inherited Variants in GBF1 are Associated with Axonal Neuropathy Caused by Golgi Fragmentation.Am J Hum Genet. 2020 Oct 1;107(4):763-777. doi: 10.1016/j.ajhg.2020.08.018. Epub 2020 Sep 15. Am J Hum Genet. 2020. PMID: 32937143 Free PMC article.
-
The Peripheral Nervous System in Amyotrophic Lateral Sclerosis: Opportunities for Translational Research.Front Neurosci. 2019 Jun 25;13:601. doi: 10.3389/fnins.2019.00601. eCollection 2019. Front Neurosci. 2019. PMID: 31293369 Free PMC article. Review.
-
Mutations in HspB1 and hereditary neuropathies.Cell Stress Chaperones. 2020 Jul;25(4):655-665. doi: 10.1007/s12192-020-01099-9. Epub 2020 Apr 16. Cell Stress Chaperones. 2020. PMID: 32301006 Free PMC article. Review.
-
HspB5/αB-crystallin increases dendritic complexity and protects the dendritic arbor during heat shock in cultured rat hippocampal neurons.Cell Mol Life Sci. 2016 Oct;73(19):3761-75. doi: 10.1007/s00018-016-2219-9. Epub 2016 Apr 16. Cell Mol Life Sci. 2016. PMID: 27085702 Free PMC article.
-
Non-Neuronal Cells Are Required to Mediate the Effects of Neuroinflammation: Results from a Neuron-Enriched Culture System.PLoS One. 2016 Jan 20;11(1):e0147134. doi: 10.1371/journal.pone.0147134. eCollection 2016. PLoS One. 2016. PMID: 26788729 Free PMC article.
References
-
- Dierick I., Irobi J., De Jonghe P., Timmerman V. Small heat shock proteins in inherited peripheral neuropathies. Ann. Med. 2005;37:413–422. doi:10.1080/07853890500296410. - DOI - PubMed
-
- Haslbeck M., Franzmann T., Weinfurtner D., Buchner J. Some like it hot: the structure and function of small heat-shock proteins. Nat. Struct. Mol. Biol. 2005;12:842–846. doi:10.1038/nsmb993. - DOI - PubMed
-
- Walter S., Buchner J. Molecular chaperones—cellular machines for protein folding. Angew. Chem. Int. Ed Engl. 2002;41:1098–1113. doi:10.1002/1521-3773(20020402)41:7<1098::AID-ANIE1098>3.0.CO;2-9. - DOI - PubMed
-
- Tang B.S., Zhao G.H., Luo W., Xia K., Cai F., Pan Q., Zhang R.X., Zhang F.F., Liu X.M., Chen B., et al. Small heat-shock protein 22 mutated in autosomal dominant Charcot-Marie-Tooth disease type 2L. Hum. Genet. 2005;116:222–224. doi:10.1007/s00439-004-1218-3. - DOI - PubMed
-
- Irobi J., Van Impe K., Seeman P., Jordanova A., Dierick I., Verpoorten N., Michalik A., De Vriendt E., Jacobs A., Van Gerwen V., et al. Hot-spot residue in small heat-shock protein 22 causes distal motor neuropathy. Nat. Genet. 2004;36:597–601. doi:10.1038/ng1328. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

