Persistent cAMP signaling by thyrotropin (TSH) receptors is not dependent on internalization
- PMID: 20538910
- PMCID: PMC2996905
- DOI: 10.1096/fj.10-161745
Persistent cAMP signaling by thyrotropin (TSH) receptors is not dependent on internalization
Abstract
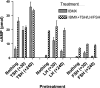
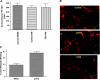
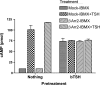
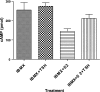
Evidence was presented that thyrotropin [thyroid-stimulating hormone (TSH)]-stimulated persistent cAMP signaling is dependent on receptor (with G-protein α subunits and adenylyl cyclase) internalization. Because it is not clear whether G proteins and adenylyl cyclase internalize with receptors, we tested whether persistent cAMP signaling by TSH receptor (TSHR) is dependent on internalization. We measured persistent TSHR signaling as an accumulation of cAMP in HEK-EM293 cells permanently expressing human TSHRs incubated with isobutylmethylxanthine for 30 min after washing the cells to remove unbound TSH, and TSHR internalization by fluorescence microscopy using Alexa-tagged TSH and binding assays using (125)I-TSH. TSHRs, but not the closely related lutropin or follitropin receptors, exhibit persistent cAMP signaling. TSHRs were not internalized by 30 min incubation with unlabeled TSH; however, expression of β-arrestin-2 promoted TSHR internalization that was inhibited by dynasore, a dynamin inhibitor. Expression of β-arrestin-2 had no effect on TSHR cAMP signaling, dynasore inhibited TSHR cAMP signaling in the absence or presence of TSHR internalization, and expression of a dominant-negative mutant dynamin, which inhibited internalization, had no effect on persistent cAMP signaling. Persistent cAMP signaling was specifically inhibited by a small molecule TSHR antagonist. We conclude that TSHRs do not have to be internalized to exhibit persistent cAMP signaling.
Figures
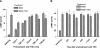
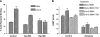
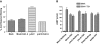
Similar articles
-
Persistent cAMP signaling by TSH receptors revealed by phosphodiesterase inhibition.Thyroid. 2013 Nov;23(11):1484-9. doi: 10.1089/thy.2013.0089. Epub 2013 Sep 20. Thyroid. 2013. PMID: 23713896 Free PMC article.
-
Persistent cAMP signaling by internalized TSH receptors occurs in thyroid but not in HEK293 cells.FASEB J. 2012 May;26(5):2043-8. doi: 10.1096/fj.11-195248. Epub 2012 Jan 30. FASEB J. 2012. PMID: 22291442
-
Thyrotropin Causes Dose-dependent Biphasic Regulation of cAMP Production Mediated by Gs and Gi/o Proteins.Mol Pharmacol. 2020 Jan;97(1):2-8. doi: 10.1124/mol.119.117382. Epub 2019 Nov 8. Mol Pharmacol. 2020. PMID: 31704717 Free PMC article.
-
β-Arrestin 1 in Thyrotropin Receptor Signaling in Bone: Studies in Osteoblast-Like Cells.Front Endocrinol (Lausanne). 2020 May 20;11:312. doi: 10.3389/fendo.2020.00312. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32508750 Free PMC article. Review.
-
Update in TSH receptor agonists and antagonists.J Clin Endocrinol Metab. 2012 Dec;97(12):4287-92. doi: 10.1210/jc.2012-3080. Epub 2012 Sep 27. J Clin Endocrinol Metab. 2012. PMID: 23019348 Free PMC article. Review.
Cited by
-
Characterizing the Interplay of Lymphocytes in Graves' Disease.Int J Mol Sci. 2023 Apr 6;24(7):6835. doi: 10.3390/ijms24076835. Int J Mol Sci. 2023. PMID: 37047805 Free PMC article. Review.
-
TSH stimulation of human thyroglobulin and thyroid peroxidase gene transcription is partially dependent on internalization.Cell Signal. 2022 Feb;90:110212. doi: 10.1016/j.cellsig.2021.110212. Epub 2021 Dec 9. Cell Signal. 2022. PMID: 34896620 Free PMC article.
-
G protein-coupled receptors: mutations and endocrine diseases.Nat Rev Endocrinol. 2011 Jun;7(6):362-72. doi: 10.1038/nrendo.2011.20. Epub 2011 Feb 8. Nat Rev Endocrinol. 2011. PMID: 21301490 Review.
-
Structural-Functional Features of the Thyrotropin Receptor: A Class A G-Protein-Coupled Receptor at Work.Front Endocrinol (Lausanne). 2017 Apr 24;8:86. doi: 10.3389/fendo.2017.00086. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 28484426 Free PMC article. Review.
-
Cell non-autonomous signaling through the conserved C. elegans glycoprotein hormone receptor FSHR-1 regulates cholinergic neurotransmission.PLoS Genet. 2024 Nov 19;20(11):e1011461. doi: 10.1371/journal.pgen.1011461. eCollection 2024 Nov. PLoS Genet. 2024. PMID: 39561202 Free PMC article.
References
-
- Hausdorff W. P., Caron M. G., Lefkowitz R. J. (1990) Turning off the signal: desensitization of β-adrenergic receptor function. FASEB J. 4, 2881–2889 - PubMed
-
- Hanyaloglu A. C., Von Zastrow M. (2008) Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu. Rev. Pharmacol. Toxicol. 48, 537–568 - PubMed
-
- Mullershausen F., Zecri F., Cetin C., Billich A., Guerini D., Seuwen K. (2009) Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat. Chem. Biol. 5, 428–434 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

