Inhibition of histone deacetylase 2 expression by elevated glucocorticoid receptor beta in steroid-resistant asthma
- PMID: 20538962
- PMCID: PMC2970859
- DOI: 10.1164/rccm.201001-0015OC
Inhibition of histone deacetylase 2 expression by elevated glucocorticoid receptor beta in steroid-resistant asthma
Abstract
Rationale: Cross-talk between glucocorticoid receptors and histone deacetylases (HDACs) under steroid-insensitive conditions has not been explored.
Objectives: To evaluate expression and interaction of HDACs with glucocorticoid receptor isoforms in bronchoalveolar lavage and peripheral blood mononuclear cells from steroid-resistant versus steroid-sensitive patients with asthma.
Methods: Expression of HDACs 1 through 11 was measured by real-time polymerase chain reaction in primary cells and in the DO11.10 cell line, designed to overexpress glucocorticoid receptor β. Glucocorticoid receptor β expression was inhibited in bronchoalveolar lavage cells by small interfering RNA. Human HDAC2 promoter fragments were cloned into a luciferase reporter vector, and transiently transfected with glucocorticoid receptor α- and β-encoding plasmids into the cells. Luciferase activity was then assayed in response to glucocorticoids.
Measurements and main results: Levels of HDAC2 mRNA, but not other histone deacetylases, were significantly decreased in bronchoalveolar lavage cells but not in peripheral blood mononuclear cells from steroid-resistant patients with asthma. Overexpression of glucocorticoid receptor β in DO11.10 cells selectively reduced HDAC2 mRNA and protein levels. Silencing of glucocorticoid receptor β in bronchoalveolar lavage cells from patients with asthma significantly increased HDAC2 mRNA. Luciferase activity assays with HDAC2 promoter reporter constructs identified two glucocorticoid-inducible regions in the HDAC2 promoter. Promoter activity was increased more than fourfold in dexamethasone-treated cells cotransfected with glucocorticoid receptor α. Cotransfection of glucocorticoid receptor β abolished this effect in a dose-dependent manner.
Conclusions: Glucocorticoid receptor β controls expression of histone deacetylase 2 by inhibiting glucocorticoid response elements in its promoter. This highlights a novel mechanism by which glucocorticoid receptor β promotes steroid insensitivity (Li et al.: J Allergy Clin Immunol 2009;123:S146; and Li et al.: J Allergy Clin Immunol 2010;125:AB104).
Figures


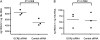

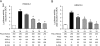
Similar articles
-
Glucocorticoid receptor β and histone deacetylase 1 and 2 expression in the airways of severe asthma.Thorax. 2012 May;67(5):392-8. doi: 10.1136/thoraxjnl-2011-200760. Epub 2011 Dec 8. Thorax. 2012. PMID: 22156779
-
Glucocorticoid receptor and histone deacetylase-2 mediate dexamethasone-induced repression of MUC5AC gene expression.Am J Respir Cell Mol Biol. 2012 Nov;47(5):637-44. doi: 10.1165/rcmb.2012-0009OC. Epub 2012 Jul 12. Am J Respir Cell Mol Biol. 2012. PMID: 22798432 Free PMC article.
-
Impact of Gene Expression Associated with Glucocorticoid-Induced Transcript 1 (GLCCI1) on Severe Asthma and Future Exacerbation.Biol Pharm Bull. 2019 Oct 1;42(10):1746-1752. doi: 10.1248/bpb.b19-00476. Epub 2019 Aug 6. Biol Pharm Bull. 2019. PMID: 31391381
-
Histone deacetylase-2 and airway disease.Ther Adv Respir Dis. 2009 Oct;3(5):235-43. doi: 10.1177/1753465809348648. Epub 2009 Oct 7. Ther Adv Respir Dis. 2009. PMID: 19812111 Review.
-
Glucocorticoid-regulated transcription factors.Pulm Pharmacol Ther. 2001;14(3):211-9. doi: 10.1006/pupt.2001.0283. Pulm Pharmacol Ther. 2001. PMID: 11448148 Review.
Cited by
-
Race is associated with differences in airway inflammation in patients with asthma.J Allergy Clin Immunol. 2017 Jul;140(1):257-265.e11. doi: 10.1016/j.jaci.2016.10.024. Epub 2017 Jan 6. J Allergy Clin Immunol. 2017. PMID: 28069248 Free PMC article. Clinical Trial.
-
Glucocorticoids, their uses, sexual dimorphisms, and diseases: new concepts, mechanisms, and discoveries.Physiol Rev. 2024 Jan 1;104(1):473-532. doi: 10.1152/physrev.00021.2023. Epub 2023 Sep 21. Physiol Rev. 2024. PMID: 37732829 Free PMC article. Review.
-
The pulmonary damage caused by smoking: A longitudinal study.Technol Health Care. 2018;26(S1):501-507. doi: 10.3233/THC-174800. Technol Health Care. 2018. PMID: 29758973 Free PMC article.
-
Gene expression profiling of asthma phenotypes demonstrates molecular signatures of atopy and asthma control.J Allergy Clin Immunol. 2016 May;137(5):1390-1397.e6. doi: 10.1016/j.jaci.2015.09.058. Epub 2016 Jan 12. J Allergy Clin Immunol. 2016. PMID: 26792209 Free PMC article.
-
Concise review: stem cell-derived erythrocytes as upcoming players in blood transfusion.Stem Cells. 2012 Aug;30(8):1587-96. doi: 10.1002/stem.1136. Stem Cells. 2012. PMID: 22644674 Free PMC article. Review.
References
-
- Hamid QA, Wenzel SE, Hauk PJ, Tsicopoulos A, Wallaert B, Lafitte JJ, Chrousos GP, Szefler SJ, Leung DY. Increased glucocorticoid receptor β in airway cells of glucocorticoid-insensitive asthma. Am J Respir Crit Care Med 1999;159:1600–1604. - PubMed
-
- Adcock IM, Ito K, Barnes PJ. Glucocorticoids: effects on gene transcription. Proc Am Thorac Soc 2004;1:247–254. - PubMed
-
- Ito K, Ito M, Elliott WM, Cosio B, Caramori G, Kon OM, Barczyk A, Hayashi S, Adcock IM, Hogg JC, et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med 2005;352:1967–1976. - PubMed
-
- Encio IJ, Detera-Wadleigh SD. The genomic structure of the human glucocorticoid receptor. J Biol Chem 1991;266:7182–7188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

