Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance
- PMID: 20538976
- PMCID: PMC2906566
- DOI: 10.1073/pnas.0914191107
Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance
Abstract
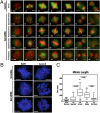
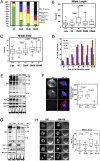
Here we show that the functional human ortholog of Greatwall protein kinase (Gwl) is the microtubule-associated serine/threonine kinase-like protein, MAST-L. This kinase promotes mitotic entry and maintenance in human cells by inhibiting protein phosphatase 2A (PP2A), a phosphatase that dephosphorylates cyclin B-Cdc2 substrates. The complete depletion of Gwl by siRNA arrests human cells in G2. When the levels of this kinase are only partially depleted, however, cells enter into mitosis with multiple defects and fail to inactivate the spindle assembly checkpoint (SAC). The ability of cells to remain arrested in mitosis by the SAC appears to be directly proportional to the amount of Gwl remaining. Thus, when Gwl is only slightly reduced, cells arrest at prometaphase. More complete depletion correlates with the premature dephosphorylation of cyclin B-Cdc2 substrates, inactivation of the SAC, and subsequent exit from mitosis with severe cytokinesis defects. These phenotypes appear to be mediated by PP2A, as they could be rescued by either a double Gwl/PP2A knockdown or by the inhibition of this phosphatase with okadaic acid. These results suggest that the balance between cyclin B-Cdc2 and PP2A must be tightly regulated for correct mitotic entry and exit and that Gwl is crucial for mediating this regulation in somatic human cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Greatwall kinase protects mitotic phosphosites from barbarian phosphatases.Proc Natl Acad Sci U S A. 2010 Jul 13;107(28):12409-10. doi: 10.1073/pnas.1006046107. Epub 2010 Jun 30. Proc Natl Acad Sci U S A. 2010. PMID: 20615940 Free PMC article. No abstract available.
References
-
- Jackman MR, Pines JN. Cyclins and the G2/M transition. Cancer Surv. 1997;29:47–73. - PubMed
-
- Bollen M, Gerlich DW, Lesage B. Mitotic phosphatases: From entry guards to exit guides. Trends Cell Biol. 2009;19:531–541. - PubMed
-
- Morgan DO. Cyclin-dependent kinases: Engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–291. - PubMed
-
- Glotzer M, Murray AW, Kirschner MW. Cyclin is degraded by the ubiquitin pathway. Nature. 1991;349:132–138. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

