Parstatin suppresses ocular neovascularization and inflammation
- PMID: 20538980
- PMCID: PMC3061514
- DOI: 10.1167/iovs.10-5576
Parstatin suppresses ocular neovascularization and inflammation
Abstract
Purpose: Parstatin is a 41-mer peptide formed by proteolytic cleavage on activation of the PAR1 receptor. The authors recently showed that parstatin is a potent inhibitor of angiogenesis. The purpose of the present study was to evaluate the therapeutic effect of parstatin on ocular neovascularization.
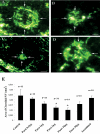
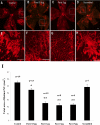
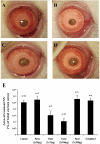
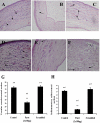
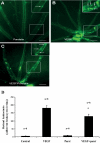
Methods: Choroidal neovascularization was generated in mice using laser-induced rupture of Bruch's membrane and was assessed after 14 days after perfusion of FITC-dextran. Oxygen-induced retinal neovascularization was established in neonatal mice by exposing them to 75% O(2) at postnatal day (P)7 for 5 days and then placing them in room air for 5 days. Evaluation was performed on P17 after staining with anti-mouse PECAM-1. The effect of parstatin was tested after intravitreal administration. The effects of subconjunctival-injected parstatin on corneal neovascularization and inflammation in rats were assessed 7 days after chemical burn-induced corneal neovascularization. Retinal leukostasis in mice was assessed after perfusion with FITC-conjugated concanavalin A.
Results: Parstatin potently inhibited choroidal neovascularization with an IC(50) of approximately 3 μg and a maximum inhibition of 59% at 10 μg. Parstatin suppressed retinal neovascularization with maximum inhibition of 60% at 3 μg. Ten-microgram and 30-μg doses appeared to be toxic to the neonatal retina. Subconjunctival parstatin inhibited corneal neovascularization, with 200 μg the most effective dose (59% inhibition). In addition, parstatin significantly inhibited corneal inflammation and VEGF-induced retinal leukostasis. In all models tested, scrambled parstatin was without any significant effect.
Conclusions: Parstatin is a potent antiangiogenic agent of ocular neovascularization and may have clinical potential in the treatment of angiogenesis-related ocular disorders.
Figures
References
-
- Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974 - PubMed
-
- Folkman J. Angiogenesis. Ann Rev Med. 2006;57:1–18 - PubMed
-
- Tolentino MJ. Current molecular understanding and future treatment strategies for pathologic ocular neovascularization. Curr Mol Med. 2009;9:973–981 - PubMed
-
- Coughlin SR. Protease-activated receptors in hemostasis, thrombosis, and vascular biology. J Thromb Haemost. 2005;3:1800–1814 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

