Suppressive effects of azithromycin on zymosan-induced production of proinflammatory mediators by human corneal epithelial cells
- PMID: 20538995
- PMCID: PMC3061501
- DOI: 10.1167/iovs.09-4992
Suppressive effects of azithromycin on zymosan-induced production of proinflammatory mediators by human corneal epithelial cells
Abstract
Purpose: In addition to its antibiotic effects, azithromycin has been noted to have anti-inflammatory activity, particularly in the context of microbial infections. This study was conducted to explore the suppressive effects of azithromycin on the production of proinflammatory mediators by human corneal epithelial cells (HCECs) stimulated by a fungal component, zymosan.
Methods: Primary HCECs were cultured from donor corneal limbal explants and grown to subconfluence. The cells were treated with toll-like receptor (TLR) 2 agonist zymosan (1-50 μg/mL) for 4 to 48 hours, with or without preincubation with azithromycin (1-50 μg/mL), TLR2 antibody, or NF-κB activation inhibitor quinazoline (NF-κB-I). The cells were subjected to total RNA extraction, reverse transcription (RT), and real-time PCR using gene expression assays. Cells treated for 48 hours were used for immunofluorescence staining and Western blot analysis, and their medium supernatants were collected for protein quantitation by immunobead assays.
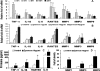
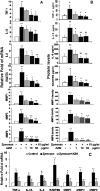
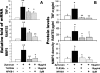
Results: The mRNA expression and protein production of proinflammatory cytokines (TNF-α and IL-1β), chemokines (IL-8 and RANTES), and matrix metalloproteinases (MMP-1, -3, and -9) by HCECs were stimulated by zymosan in a concentration-dependent manner, with peak levels noted at 4 hours. These stimulated levels of proinflammatory mediators by zymosan were significantly inhibited by TLR2 antibody, NF-κB-I, or azithromycin, which blocked zymosan-induced NF-κB activation as determined by p65 protein nuclear translocation.
Conclusions: These findings demonstrated that the fungal component zymosan induces proinflammatory responses through TLR2 and NF-κB signaling pathways, whereas azithromycin suppresses its stimulation by blocking NF-κB activation in HCECs, suggesting the potential efficacy of this antibiotic for treating ocular surface inflammatory disorders.
Figures

References
-
- Luchs J. Efficacy of topical azithromycin ophthalmic solution 1% in the treatment of posterior blepharitis. Adv Ther. 2008;25:858–870 - PubMed
-
- Cigana C, Nicolis E, Pasetto M, Assael BM, Melotti P. Anti-inflammatory effects of azithromycin in cystic fibrosis airway epithelial cells. Biochem Biophys Res Commun. 2006;350:977–982 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

