Transient receptor potential ion channels V4 and A1 contribute to pancreatitis pain in mice
- PMID: 20539005
- PMCID: PMC2950679
- DOI: 10.1152/ajpgi.00433.2009
Transient receptor potential ion channels V4 and A1 contribute to pancreatitis pain in mice
Abstract
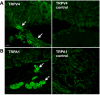
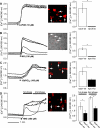
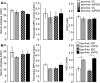
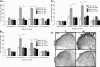
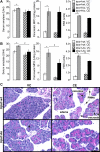
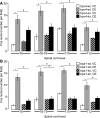
The mechanisms of pancreatic pain, a cardinal symptom of pancreatitis, are unknown. Proinflammatory agents that activate transient receptor potential (TRP) channels in nociceptive neurons can cause neurogenic inflammation and pain. We report a major role for TRPV4, which detects osmotic pressure and arachidonic acid metabolites, and TRPA1, which responds to 4-hydroxynonenal and cyclopentenone prostaglandins, in pancreatic inflammation and pain in mice. Immunoreactive TRPV4 and TRPA1 were detected in pancreatic nerve fibers and in dorsal root ganglia neurons innervating the pancreas, which were identified by retrograde tracing. Agonists of TRPV4 and TRPA1 increased intracellular Ca(2+) concentration ([Ca(2+)](i)) in these neurons in culture, and neurons also responded to the TRPV1 agonist capsaicin and are thus nociceptors. Intraductal injection of TRPV4 and TRPA1 agonists increased c-Fos expression in spinal neurons, indicative of nociceptor activation, and intraductal TRPA1 agonists also caused pancreatic inflammation. The effects of TRPV4 and TRPA1 agonists on [Ca(2+)](i), pain and inflammation were markedly diminished or abolished in trpv4 and trpa1 knockout mice. The secretagogue cerulein induced pancreatitis, c-Fos expression in spinal neurons, and pain behavior in wild-type mice. Deletion of trpv4 or trpa1 suppressed c-Fos expression and pain behavior, and deletion of trpa1 attenuated pancreatitis. Thus TRPV4 and TRPA1 contribute to pancreatic pain, and TRPA1 also mediates pancreatic inflammation. Our results provide new information about the contributions of TRPV4 and TRPA1 to inflammatory pain and suggest that channel antagonists are an effective therapy for pancreatitis, when multiple proinflammatory agents are generated that can activate and sensitize these channels.
Figures






References
-
- Alessandri-Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB, Levine JD. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron 39: 497–511, 2003 - PubMed
-
- Aleynik SI, Leo MA, Aleynik MK, Lieber CS. Alcohol-induced pancreatic oxidative stress: protection by phospholipid repletion. Free Radic Biol Med 26: 609–619, 1999 - PubMed
-
- Amadesi S, Nie J, Vergnolle N, Cottrell GS, Grady EF, Trevisani M, Manni C, Geppetti P, McRoberts JA, Ennes H, Davis JB, Mayer EA, Bunnett NW. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J Neurosci 24: 4300–4312, 2004 - PMC - PubMed
-
- Anaparthy R, Pasricha PJ. Pain and chronic pancreatitis: is it the plumbing or the wiring? Curr Gastroenterol Rep 10: 101–106, 2008 - PubMed
-
- Andre E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, Creminon C, Vaksman N, Nassini R, Civelli M, Baraldi PG, Poole DP, Bunnett NW, Geppetti P, Patacchini R. Cigarette smoke-induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest 118: 2574–2582, 2008 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

