Nitrates and nitrites in the treatment of ischemic cardiac disease
- PMID: 20539102
- PMCID: PMC2885014
- DOI: 10.1097/CRD.0b013e3181c8e14a
Nitrates and nitrites in the treatment of ischemic cardiac disease
Abstract
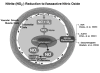
The organic nitrite, amyl of nitrite, was initially used as a therapeutic agent in the treatment of angina pectoris, but was replaced over a decade later by the organic nitrate, nitroglycerin (NTG), due to the ease of administration and longer duration of action. The administration of organic nitrate esters, such as NTG, continues to be used in the treatment of angina pectoris and heart failure since the birth of modern pharmacology. Their clinical effectiveness is due to vasodilator activity in large veins and arteries through an as yet unidentified method of delivering nitric oxide (NO), or a NO-like compound. The major drawback is the development of tolerance with NTG, and the duration and route of administration with amyl of nitrite. Although the nitrites are no longer used in the treatment of hypertension or ischemic heart disease, the nitrite anion has recently been discovered to possess novel pharmacologic actions, such as modulating hypoxic vasodilation, and providing cytoprotection in ischemia-reperfusion injury. Although the actions of these 2 similar chemical classes (nitrites and organic nitrates) have often been considered to be alike, we still do not understand their mechanism of action. Finally, the nitrite anion, either from sodium nitrite or an intermediate NTG form, may act as a storage form for NO and provide support for investigating the use of these agents in the treatment of ischemic cardiovascular states. We review what is presently known about the use of nitrates and nitrites including the historical, current, and potential uses of these agents, and their mechanisms of action.
Figures
References
-
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349:1498–1504. - PubMed
-
- Yach D, Hawkes C, Gould CL, et al. The global burden of chronic diseases: overcoming impediments to prevention and control. JAMA. 2004;291:2616–2622. - PubMed
-
- Brunton TL. On the use of nitrite of amyl in angina pectoris. Lancet. 1867;2:97–98.
-
- Murrell W. Nitro-Glycerine in angina pectoris. Lancet. 1879;1:80–81.
-
- Contro S, Haring OM, Goldstein W. Paradoxic action of amyl nitrite in coronary patients. Circulation. 1952;6:250–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

