Normal levels of p27 are necessary for somite segmentation and determining pronephric organ size
- PMID: 20539739
- PMCID: PMC2878748
- DOI: 10.4161/org.5.4.9973
Normal levels of p27 are necessary for somite segmentation and determining pronephric organ size
Abstract
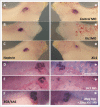
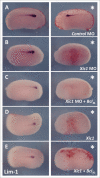
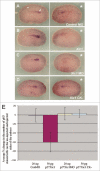
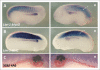
The Xenopus laevis cyclin dependent kinase inhibitor p27(Xic1) has been shown to be involved in exit from the cell cycle and differentiation of cells into a quiescent state in the nervous system, muscle tissue, heart and retina. We show that p27(Xic1) is expressed in the developing kidney in the nephrostomal regions. Using overexpression and morpholino oligonucleotide (MO) knock-down approaches we show normal levels of p27(Xic1) regulate pronephros organ size by regulating cell cycle exit. Knock-down of p27(Xic1) expression using a MO prevented myogenesis, as previously reported; an effect that subsequently inhibits pronephrogenesis. Furthermore, we show that normal levels of p27(Xic1) are required for somite segmentation also through its cell cycle control function. Finally, we provide evidence to suggest correct paraxial mesoderm segmentation is not necessary for pronephric induction in the intermediate mesoderm. These results indicate novel developmental roles for p27(Xic1), and reveal its differentiation function is not universally utilised in all developing tissues.
Keywords: cell cycle; morpholino; organ size; overexpression; p27Xic1; pronephros; xenopus.
Figures




Similar articles
-
Depletion of the cell-cycle inhibitor p27(Xic1) impairs neuronal differentiation and increases the number of ElrC(+) progenitor cells in Xenopus tropicalis.Mech Dev. 2003 May;120(5):607-16. doi: 10.1016/s0925-4773(03)00010-8. Mech Dev. 2003. PMID: 12782277
-
A single cdk inhibitor, p27Xic1, functions beyond cell cycle regulation to promote muscle differentiation in Xenopus.Development. 2003 Jan;130(1):71-83. doi: 10.1242/dev.00180. Development. 2003. PMID: 12441292
-
Cardiac differentiation in Xenopus requires the cyclin-dependent kinase inhibitor, p27Xic1.Cardiovasc Res. 2008 Aug 1;79(3):436-47. doi: 10.1093/cvr/cvn105. Epub 2008 Apr 27. Cardiovasc Res. 2008. PMID: 18442987 Free PMC article.
-
Towards a molecular anatomy of the Xenopus pronephric kidney.Int J Dev Biol. 1999;43(5):381-95. Int J Dev Biol. 1999. PMID: 10535314 Review.
-
Specification and segmentation of the paraxial mesoderm.Anat Embryol (Berl). 1994 Apr;189(4):275-305. doi: 10.1007/BF00190586. Anat Embryol (Berl). 1994. PMID: 8074321 Review.
References
-
- Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin) 4th ed. New York: Garland Publishing, Inc.; 1994.
-
- Saxén L. Organogesis of the kidney. Cambridge: Cambridge University Press; 1987.
-
- Vize PD, Jones EA, Pfister R. Development of the Xenopus pronephric system. Dev Biol. 1995;171:531–540. - PubMed
-
- Brändli AW. Towards a molecular anatomy of the Xenopus pronephric kidney. Int J Dev Biol. 1999;43:381–395. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
