Allosteric regulation of protease activity by small molecules
- PMID: 20539873
- PMCID: PMC4356121
- DOI: 10.1039/c003913f
Allosteric regulation of protease activity by small molecules
Abstract
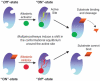
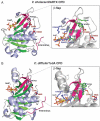
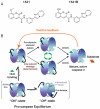
Proteases regulate a plethora of biological processes. Because they irreversibly cleave peptide bonds, the activity of proteases is strictly controlled. While there are many ways to regulate protease activity, an emergent mechanism is the modulation of protease function by small molecules acting at allosteric sites. This mode of regulation holds the potential to allow for the specific and temporal control of a given biological process using small molecules. These compounds also serve as useful tools for studying protein dynamics and function. This review highlights recent advances in identifying and characterizing natural and synthetic small molecule allosteric regulators of proteases and discusses their utility in studies of protease function, drug discovery and protein engineering.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

