Bivalent diketopiperazine-based tropomysin receptor kinase C (TrkC) antagonists
- PMID: 20540510
- PMCID: PMC2907676
- DOI: 10.1021/jm100148d
Bivalent diketopiperazine-based tropomysin receptor kinase C (TrkC) antagonists
Abstract
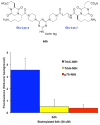
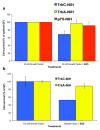
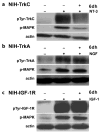
Bivalent molecules containing two beta-turn mimics with side chains that correspond to hot-spots on the neurotrophin NT-3 were prepared. Binding assays showed the mimetics to be selective TrkC ligands, and biological assays showed one mimetic to be an antagonist of the TrkC receptor.
Figures

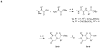
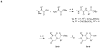
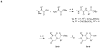
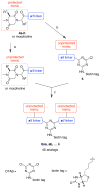
Similar articles
-
Bivalent peptidomimetic ligands of TrkC are biased agonists and selectively induce neuritogenesis or potentiate neurotrophin-3 trophic signals.ACS Chem Biol. 2009 Sep 18;4(9):769-81. doi: 10.1021/cb9001415. ACS Chem Biol. 2009. PMID: 19735123 Free PMC article.
-
A peptidomimetic of NT-3 acts as a TrkC antagonist.Peptides. 2009 Oct;30(10):1833-9. doi: 10.1016/j.peptides.2009.07.015. Epub 2009 Jul 30. Peptides. 2009. PMID: 19647025 Free PMC article.
-
Combinatorial assembly of small molecules into bivalent antagonists of TrkC or TrkA receptors.PLoS One. 2014 Mar 6;9(3):e89617. doi: 10.1371/journal.pone.0089617. eCollection 2014. PLoS One. 2014. PMID: 24603864 Free PMC article.
-
[The role of TrkC receptor and neurotrophin 3 in the development and function of neural cells].Postepy Hig Med Dosw (Online). 2005;59:517-22. Postepy Hig Med Dosw (Online). 2005. PMID: 16258418 Review. Polish.
-
Crystal Structures of Neurotrophin Receptors Kinase Domain.Vitam Horm. 2017;104:1-18. doi: 10.1016/bs.vh.2016.10.001. Epub 2016 Nov 29. Vitam Horm. 2017. PMID: 28215291 Review.
Cited by
-
Targeted PDT agent eradicates TrkC expressing tumors via photodynamic therapy (PDT).Mol Pharm. 2015 Jan 5;12(1):212-22. doi: 10.1021/mp5005564. Epub 2014 Dec 9. Mol Pharm. 2015. PMID: 25487316 Free PMC article.
-
Neuroprotection: Pro-survival and Anti-neurotoxic Mechanisms as Therapeutic Strategies in Neurodegeneration.Front Cell Neurosci. 2019 Jun 6;13:231. doi: 10.3389/fncel.2019.00231. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31244606 Free PMC article. Review.
-
The diketopiperazine-fused tetrahydro-β-carboline scaffold as a model peptidomimetic with an unusual α-turn secondary structure.Beilstein J Org Chem. 2013;9:147-54. doi: 10.3762/bjoc.9.17. Epub 2013 Jan 22. Beilstein J Org Chem. 2013. PMID: 23399789 Free PMC article.
-
Developing bivalent ligands to target CUG triplet repeats, the causative agent of myotonic dystrophy type 1.J Med Chem. 2013 Dec 12;56(23):9471-9481. doi: 10.1021/jm400794z. Epub 2013 Nov 21. J Med Chem. 2013. PMID: 24188018 Free PMC article.
-
Low molecular weight, non-peptidic agonists of TrkA receptor with NGF-mimetic activity.Cell Death Dis. 2012 Jul 5;3(7):e339. doi: 10.1038/cddis.2012.80. Cell Death Dis. 2012. PMID: 22764098 Free PMC article.
References
-
- Skaper SD. The biology of neurotrophins, signalling pathways, and functional peptide mimetics of neurotrophins and their receptors. CNS & Neurological Disorders: Drug Targets. 2008;7:46–62. - PubMed
-
- Ivanisevic L, Saragovi HU. The Neurotrophins. In: Kastin AJ, editor. Handbook of Biologically Active Peptides. Elsevier; 2006. pp. 1407–1413.
-
- Pollack SJ, Harper SJ. Small molecule Trk receptor agonists and other neurotrophic factor mimetics. Current Drug Targets: CNS & Neurological Disorders. 2002;1:59–80. - PubMed
-
- Friedman WJ, Greene LA. Neurotrophin signaling via Trks and p75. Exp Cell Res. 1999;253:131–142. - PubMed
-
- Feng Y, Pattarawarapan M, Wang Z, Burgess K. Solid-phase SN2 macrocyclization reactions to form β-turn mimics. Org Lett. 1999;1:121–124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

