Unexpected hormonal activity of a catechol equine estrogen metabolite reveals reversible glutathione conjugation
- PMID: 20540524
- PMCID: PMC2941764
- DOI: 10.1021/tx100129h
Unexpected hormonal activity of a catechol equine estrogen metabolite reveals reversible glutathione conjugation
Abstract
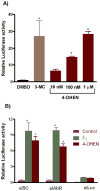
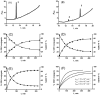
4-Hydroxyequilenin (4-OHEN) is a major phase I metabolite of the equine estrogens present in widely prescribed hormone replacement formulations. 4-OHEN is autoxidized to an electrophilic o-quinone that has been shown to redox cycle, generating ROS, and to covalently modify proteins and DNA and thus potentially to act as a chemical carcinogen. To establish the ability of 4-OHEN to act as a hormonal carcinogen at the estrogen receptor (ER), estrogen responsive gene expression and proliferation were studied in ER(+) breast cancer cells. Recruitment by 4-OHEN of ER to estrogen responsive elements (ERE) of DNA in MCF-7 cells was also studied and observed. 4-OHEN was a potent estrogen, with additional weak activity associated with binding to the arylhydrocarbon receptor (AhR). The potency of 4-OHEN toward classical ERalpha mediated activity was unexpected given the reported rapid autoxidation and trapping of the resultant quinone by GSH. Addition of thiols to cell cultures did not attenuate the estrogenic activity of 4-OHEN, and preformed thiol conjugates added to cell incubations only marginally reduced ERE-luciferase induction. On reaction of the 4OHEN-GSH conjugate with NADPH, 4-OHEN was observed to be regenerated at a rate dependent upon NADPH concentration, indicating that intracellular nonenzymatic and enzymatic regeneration of 4-OHEN accounts for the observed estrogenic activity of 4-OHEN. 4-OHEN is therefore capable of inducing chemical and hormonal pathways that may contribute to estrogen-dependent carcinogenesis, and trapping by cellular thiols does not provide a mechanism of termination of these pathways.
Figures





Comment in
-
In this issue. Estrogen double whammy.Chem Res Toxicol. 2010 Aug 16;23(8):1303-4. doi: 10.1021/tx1002029. Chem Res Toxicol. 2010. PMID: 20707404 No abstract available.
Similar articles
-
Estrogen Receptor {alpha} Enhances the Rate of Oxidative DNA Damage by Targeting an Equine Estrogen Catechol Metabolite to the Nucleus.J Biol Chem. 2009 Mar 27;284(13):8633-42. doi: 10.1074/jbc.M807860200. Epub 2009 Jan 21. J Biol Chem. 2009. PMID: 19158089 Free PMC article.
-
Redox cycling of catechol estrogens generating apurinic/apyrimidinic sites and 8-oxo-deoxyguanosine via reactive oxygen species differentiates equine and human estrogens.Chem Res Toxicol. 2010 Aug 16;23(8):1365-73. doi: 10.1021/tx1001282. Chem Res Toxicol. 2010. PMID: 20509668 Free PMC article.
-
Inhibition of glutathione S-transferase activity by the quinoid metabolites of equine estrogens.Chem Res Toxicol. 1998 Jul;11(7):758-65. doi: 10.1021/tx9702190. Chem Res Toxicol. 1998. PMID: 9671538
-
Molecular mechanisms of estrogen action: selective ligands and receptor pharmacology.J Steroid Biochem Mol Biol. 2000 Nov 30;74(5):279-85. doi: 10.1016/s0960-0760(00)00104-7. J Steroid Biochem Mol Biol. 2000. PMID: 11162936 Review.
-
Potential mechanisms of estrogen quinone carcinogenesis.Chem Res Toxicol. 2008 Jan;21(1):93-101. doi: 10.1021/tx700191p. Epub 2007 Dec 4. Chem Res Toxicol. 2008. PMID: 18052105 Free PMC article. Review.
Cited by
-
Genotoxicity of ortho-quinones: reactive oxygen species versus covalent modification.Toxicol Res (Camb). 2017;6(6):740-754. doi: 10.1039/C7TX00223H. Epub 2017 Sep 6. Toxicol Res (Camb). 2017. PMID: 29527287 Free PMC article.
-
Formation and Biological Targets of Quinones: Cytotoxic versus Cytoprotective Effects.Chem Res Toxicol. 2017 Jan 17;30(1):13-37. doi: 10.1021/acs.chemrestox.6b00256. Epub 2016 Sep 29. Chem Res Toxicol. 2017. PMID: 27617882 Free PMC article.
References
-
- Liehr JG. Genotoxic effects of estrogens. Mutation Research. 1990;238:269–276. - PubMed
-
- Colditz GA. Relationship between estrogen levels, use of hormone replacement therapy, and breast cancer. J Natl Cancer Inst. 1998;90:814–823. - PubMed
-
- Feigelson HS, Henderson BE. Estrogens and breast cancer. Carcinogenesis. 1996;17:2279–2284. - PubMed
-
- Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–282. - PubMed
-
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women's health initiative randomized controlled trial. J Am Med Assoc. 2002;288:321–333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

