Improved 18F labeling of peptides with a fluoride-aluminum-chelate complex
- PMID: 20540570
- PMCID: PMC2913283
- DOI: 10.1021/bc100137x
Improved 18F labeling of peptides with a fluoride-aluminum-chelate complex
Abstract
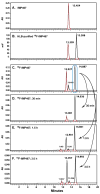
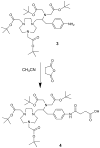
We reported previously the feasibility to radiolabel peptides with fluorine-18 ((18)F) using a rapid one-pot method that first mixes (18)F(-) with Al(3+) and then binds the (Al(18)F)(2+) complex to a NOTA ligand on the peptide. In this report, we examined several new NOTA ligands and determined how temperature, reaction time, and reagent concentration affected the radiolabeling yield. Four structural variations of the NOTA ligand had isolated radiolabeling yields ranging from 5.8% to 87% under similar reaction conditions. All of the Al(18)F NOTA complexes were stable in vitro in human serum, and those that were tested in vivo also were stable. The radiolabeling reactions were performed at 100 degrees C, and the peptides could be labeled in as little as 5 min. The IMP467 peptide could be labeled up to 115 GBq/micromol (3100 Ci/mmol), with a total reaction and purification time of 30 min without chromatographic purification.
Figures




Similar articles
-
Optimized labeling of NOTA-conjugated octreotide with F-18.Tumour Biol. 2012 Apr;33(2):427-34. doi: 10.1007/s13277-011-0250-x. Epub 2011 Oct 19. Tumour Biol. 2012. PMID: 22009690 Free PMC article.
-
In vitro and in vivo evaluation of the effects of aluminum [¹⁸F]fluoride radiolabeling on an integrin αvβ₆-specific peptide.Nucl Med Biol. 2014 Jan;41(1):43-50. doi: 10.1016/j.nucmedbio.2013.09.009. Epub 2013 Oct 8. Nucl Med Biol. 2014. PMID: 24267053
-
A novel facile method of labeling octreotide with (18)F-fluorine.J Nucl Med. 2010 Mar;51(3):454-61. doi: 10.2967/jnumed.109.066902. Epub 2010 Feb 11. J Nucl Med. 2010. PMID: 20150268 Free PMC article.
-
A Comprehensive Review of Non-Covalent Radiofluorination Approaches Using Aluminum [18F]fluoride: Will [18F]AlF Replace 68Ga for Metal Chelate Labeling?Molecules. 2019 Aug 7;24(16):2866. doi: 10.3390/molecules24162866. Molecules. 2019. PMID: 31394799 Free PMC article. Review.
-
Al(18) F labeling of peptides and proteins.J Labelled Comp Radiopharm. 2014 Apr;57(4):219-23. doi: 10.1002/jlcr.3161. Epub 2014 Jan 10. J Labelled Comp Radiopharm. 2014. PMID: 24408125 Review.
Cited by
-
Radiofluorination of a pre-formed gallium(III) aza-macrocyclic complex: towards next-generation positron emission tomography (PET) imaging agents.Chemistry. 2015 Mar 16;21(12):4688-94. doi: 10.1002/chem.201405812. Epub 2015 Feb 4. Chemistry. 2015. PMID: 25652736 Free PMC article.
-
Biological Evaluation of [18F]AlF-NOTA-NSC-GLU as a Positron Emission Tomography Tracer for Hepatocellular Carcinoma.Front Chem. 2021 Apr 16;9:630452. doi: 10.3389/fchem.2021.630452. eCollection 2021. Front Chem. 2021. PMID: 33937189 Free PMC article.
-
First 18F-Labeled Pentixafor-Based Imaging Agent for PET Imaging of CXCR4 Expression In Vivo.Tomography. 2016 Jun;2(2):85-93. doi: 10.18383/j.tom.2016.00130. Tomography. 2016. PMID: 30042959 Free PMC article.
-
Stability analysis of glutamic acid linked peptides coupled to NOTA through different chemical linkages.Mol Pharm. 2014 Nov 3;11(11):3867-74. doi: 10.1021/mp400706q. Epub 2014 Feb 24. Mol Pharm. 2014. PMID: 24533430 Free PMC article.
-
Optimized labeling of NOTA-conjugated octreotide with F-18.Tumour Biol. 2012 Apr;33(2):427-34. doi: 10.1007/s13277-011-0250-x. Epub 2011 Oct 19. Tumour Biol. 2012. PMID: 22009690 Free PMC article.
References
-
- Miller PW, Long NJ, Vilar R, Gee AD. Synthesis of 11C, 18F, 15O, and 13N radiolabels for positron emission tomography. Angew Chem Int Ed. 2008;47:8998–9033. - PubMed
-
- Wester HJ, Schottelius M. Fluorine-18 labeling of peptides and proteins. In: Schubiger PA, Lehmann L, Friebe M, editors. PET chemistry-the driving force in molecular imaging. 2007. pp. 79–111. - PubMed
-
- Cai L, Lu S, Pike VW. Chemistry with [18F]fluoride ion. Eur J Org Chem. 2008:2853–2873.
-
- Mamat C, Ramenda T, Weust FR. Recent applications of click chemistry for the synthesis of radiotracers for molecular imaging. Mini-Rev Org Chem. 2009;6:21–34.
-
- Schirrmacher E, Wängler B, Cypryk M, Bradtmöller G, Schäfer M, Eisenhut M, Jurkschat K, Schirrmacher R. Synthesis of p-(di-tert-butyl[18F]fluorosilyl)benzaldehyde ([18F] SiFA-A) with high specific activity by isotopic exchange: a convenient labeling synthon for the 18F-labeling of N-amino-oxy derivatized peptides. Bioconjugate Chem. 2007;18:2085–2089. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

