An in vivo RNA interference screen identifies gene networks controlling Drosophila melanogaster blood cell homeostasis
- PMID: 20540764
- PMCID: PMC2891661
- DOI: 10.1186/1471-213X-10-65
An in vivo RNA interference screen identifies gene networks controlling Drosophila melanogaster blood cell homeostasis
Abstract
Background: In metazoans, the hematopoietic system plays a key role both in normal development and in defense of the organism. In Drosophila, the cellular immune response involves three types of blood cells: plasmatocytes, crystal cells and lamellocytes. This last cell type is barely present in healthy larvae, but its production is strongly induced upon wasp parasitization or in mutant contexts affecting larval blood cell homeostasis. Notably, several zygotic mutations leading to melanotic mass (or "tumor") formation in larvae have been associated to the deregulated differentiation of lamellocytes. To gain further insights into the gene regulatory network and the mechanisms controlling larval blood cell homeostasis, we conducted a tissue-specific loss of function screen using hemocyte-specific Gal4 drivers and UAS-dsRNA transgenic lines.
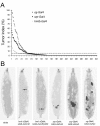
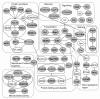
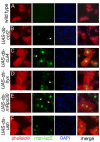
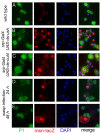
Results: By targeting around 10% of the Drosophila genes, this in vivo RNA interference screen allowed us to recover 59 melanotic tumor suppressor genes. In line with previous studies, we show that melanotic tumor formation is associated with the precocious differentiation of stem-cell like blood progenitors in the larval hematopoietic organ (the lymph gland) and the spurious differentiation of lamellocytes. We also find that melanotic tumor formation can be elicited by defects either in the fat body, the embryo-derived hemocytes or the lymph gland. In addition, we provide a definitive confirmation that lymph gland is not the only source of lamellocytes as embryo-derived plasmatocytes can differentiate into lamellocytes either upon wasp infection or upon loss of function of the Friend of GATA cofactor U-shaped.
Conclusions: In this study, we identify 55 genes whose function had not been linked to blood cell development or function before in Drosophila. Moreover our analyses reveal an unanticipated plasticity of embryo-derived plasmatocytes, thereby shedding new light on blood cell lineage relationship, and pinpoint the Friend of GATA transcription cofactor U-shaped as a key regulator of the plasmatocyte to lamellocyte transformation.
Figures


References
-
- Tepass U, Fessler LI, Aziz A, Hartenstein V. Embryonic origin of hemocytes and their relationship to cell death in Drosophila. Development. 1994;120:1829–1837. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

