Commensal microflora induce host defense and decrease bacterial translocation in burn mice through toll-like receptor 4
- PMID: 20540783
- PMCID: PMC2901327
- DOI: 10.1186/1423-0127-17-48
Commensal microflora induce host defense and decrease bacterial translocation in burn mice through toll-like receptor 4
Abstract
Background: Major burn is associated with decreased gut barrier function and increased bacterial translocation (BT). This study is to investigate whether commensal microflora induce host defense and decrease BT in burn mice.
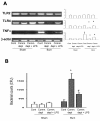
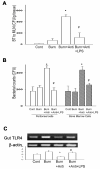
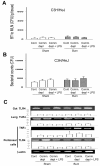
Methods: First, we treated Wild type (WT) mice with antibiotics in drinking water for 4 weeks to deplete gut commensal microflora. At week 3, drinking water was supplemented with lipopolysaccharide (LPS); a ligand for TLR4, to trigger TLRs in gut. The intestinal permeability, glutathione level, NF-kappaB DNA-binding activity, TLR4 expression of intestinal mucosa, BT to mesenteric lymph nodes (MLNs), and bacterial killing activity of peritoneal cells were measured after thermal injury. Second, lung of animals were harvested for MPO activity and TNFalpha mRNA expression assay. Third, WT animals were treated with oral antibiotics with or without LPS supplement after burn. At 48 hr after burn, TLR4 expression of intestinal mucosa and bacterial killing activity of cells were examined. Finally, bacterial killing activity and BT to MLNs after thermal injury in C3H/HeJ (TLR4 mutant) mice were measured.
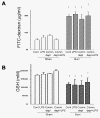
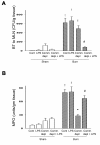
Results: Burn induced BT to MLNs in WT mice. Commensal depletion decreased TLR4 expression as well as NF-kappaB activation of intestine, myeloperoxidase (MPO) activity as well as TNFalpha expression of lung, and bacterial killing activity of peritoneal cells. Oral LPS supplement markedly reduced 81% of burn-induced BT and increased TLR4 expression, MPO activity of lung, as well as bacterial killing activity of peritoneal cells. LPS supplement did not change BT or bacterial killing activity in C3H/HeJ mice.
Conclusions: Collectively, commensal microflora induce TLR4 expression of intestine and bacterial killing activity of inflammatory cells in burn. TLR4 ligand increases bacterial killing activity and decreases burn-induced BT. Taken together with the abolition of LPS effect in TLR4 mutant mice, we conclude that commensal microflora induce host defense and decrease bacterial translocation in burn mice through toll-like receptor 4.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

