Homeobox A7 increases cell proliferation by up-regulation of epidermal growth factor receptor expression in human granulosa cells
- PMID: 20540809
- PMCID: PMC2904782
- DOI: 10.1186/1477-7827-8-61
Homeobox A7 increases cell proliferation by up-regulation of epidermal growth factor receptor expression in human granulosa cells
Abstract
Background: Homeobox (HOX) genes encode transcription factors, which regulate cell proliferation, differentiation, adhesion, and migration. The deregulation of HOX genes is frequently associated with human reproductive system disorders. However, knowledge regarding the role of HOX genes in human granulosa cells is limited.
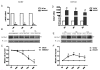
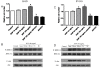
Methods: To determine the role of HOXA7 in the regulation and associated mechanisms of cell proliferation in human granulosa cells, HOXA7 and epidermal growth factor receptor (EGFR) expressions were examined in primary granulosa cells (hGCs), an immortalized human granulosa cell line, SVOG, and a granulosa tumor cell line, KGN, by real-time PCR and Western blotting. To manipulate the expression of HOXA7, the HOXA7 specific siRNA was used to knockdown HOXA7 in KGN. Conversely, HOXA7 was overexpressed in SVOG by transfection with the pcDNA3.1-HOAX7 vector. Cell proliferation was measured by the MTT assay.
Results: Our results show that HOXA7 and EGFR were overexpressed in KGN cells compared to hGCs and SVOG cells. Knockdown of HOXA7 in KGN cells significantly decreased cell proliferation and EGFR expression. Overexpression of HOXA7 in SVOG cells significantly promoted cell growth and EGFR expression. Moreover, the EGF-induced KGN proliferation was abrogated, and the activation of downstream signaling was diminished when HOXA7 was knocked down. Overexpression of HOXA7 in SVOG cells had an opposite effect.
Conclusions: Our present study reveals a novel mechanistic role for HOXA7 in modulating granulosa cell proliferation via the regulation of EGFR. This finding contributes to the knowledge of the pro-proliferation effect of HOXA7 in granulosa cell growth and differentiation.
Figures
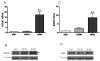
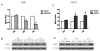
Similar articles
-
Transforming growth factor alpha (TGFα) regulates granulosa cell tumor (GCT) cell proliferation and migration through activation of multiple pathways.PLoS One. 2012;7(11):e48299. doi: 10.1371/journal.pone.0048299. Epub 2012 Nov 14. PLoS One. 2012. PMID: 23155381 Free PMC article.
-
Cell type- and stage-specific changes in HOXA7 protein expression in human ovarian folliculogenesis: possible role of GDF-9.Differentiation. 2006 Feb;74(1):1-10. doi: 10.1111/j.1432-0436.2006.00053.x. Differentiation. 2006. PMID: 16466395
-
Homeobox A7 stimulates breast cancer cell proliferation by up-regulating estrogen receptor-alpha.Biochem Biophys Res Commun. 2013 Nov 1;440(4):652-7. doi: 10.1016/j.bbrc.2013.09.121. Epub 2013 Oct 4. Biochem Biophys Res Commun. 2013. PMID: 24099775
-
HOX cofactors expression and regulation in the human ovary.Reprod Biol Endocrinol. 2008 Oct 30;6:49. doi: 10.1186/1477-7827-6-49. Reprod Biol Endocrinol. 2008. PMID: 18973687 Free PMC article.
-
The release of EGF domain from EGF-like factors by a specific cleavage enzyme activates the EGFR-MAPK3/1 pathway in both granulosa cells and cumulus cells during the ovulation process.J Reprod Dev. 2012;58(5):510-4. doi: 10.1262/jrd.2012-056. J Reprod Dev. 2012. PMID: 23124701 Review.
Cited by
-
The loss of Hoxa5 function causes estrous acyclicity and ovarian epithelial inclusion cysts.Endocrinology. 2012 Mar;153(3):1484-97. doi: 10.1210/en.2011-1766. Epub 2012 Feb 7. Endocrinology. 2012. PMID: 22315454 Free PMC article.
-
Ovarian Function Restoration Using Autologous Platelet-Rich Plasma.Reprod Med Biol. 2025 Jul 16;24(1):e12666. doi: 10.1002/rmb2.12666. eCollection 2025 Jan-Dec. Reprod Med Biol. 2025. PMID: 40678564 Free PMC article. Review.
-
Genome-wide pathway analysis in major depressive disorder.J Mol Neurosci. 2013 Oct;51(2):428-36. doi: 10.1007/s12031-013-0047-z. Epub 2013 Jun 23. J Mol Neurosci. 2013. PMID: 23794217
-
Transforming growth factor alpha (TGFα) regulates granulosa cell tumor (GCT) cell proliferation and migration through activation of multiple pathways.PLoS One. 2012;7(11):e48299. doi: 10.1371/journal.pone.0048299. Epub 2012 Nov 14. PLoS One. 2012. PMID: 23155381 Free PMC article.
-
Multichannel Recovery Potential with Activated Autologous Intraovarian Platelet-Rich Plasma and Its Derivatives.Medicines (Basel). 2023 Jul 3;10(7):40. doi: 10.3390/medicines10070040. Medicines (Basel). 2023. PMID: 37505061 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

