The effect of oral anthelmintics on the survivorship and re-feeding frequency of anthropophilic mosquito disease vectors
- PMID: 20540931
- PMCID: PMC2939250
- DOI: 10.1016/j.actatropica.2010.06.001
The effect of oral anthelmintics on the survivorship and re-feeding frequency of anthropophilic mosquito disease vectors
Abstract
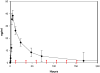
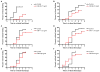
In the Tropics, there is substantial temporal and spatial overlap of diseases propagated by anthropophilic mosquito vectors (such as malaria and dengue) and human helminth diseases (such as onchocerciasis and lymphatic filariasis) that are treated though mass drug administrations (MDA). This overlap will result in mosquito vectors imbibing significant quantities of these drugs when they blood feed on humans. Since many anthelmintic drugs have broad anti-invertebrate effects, the possibility of combined helminth control and mosquito-borne disease control through MDA is apparent. It has been previously shown that ivermectin can reduce mosquito survivorship when administered in a blood meal, but more detailed examinations are needed if MDA is to ever be developed into a tool for malaria or dengue control. We examined concentrations of drugs that follow human pharmacokinetics after MDA and that matched with mosquito feeding times, for effects against the anthropophilic mosquito vectors Anopheles gambiae s.s. and Aedes aegypti. Ivermectin was the only human-approved MDA drug we tested that affected mosquito survivorship, and only An. gambiae s.s. were affected at concentrations respecting human pharmacokinetics at indicated doses. Ivermectin also delayed An. gambiae s.s. re-feeding frequency and defecation rates, and two successive ivermectin-spiked blood meals following human pharmacokinetic concentrations compounded mortality effects compared to controls. These findings suggest that ivermectin MDA in Africa may be used to decrease malaria transmission if MDAs were administered more frequently. Such a strategy would broaden the current scope of polyparasitism control already afforded by MDAs, and which is needed in many African villages simultaneously burdened by many parasitic diseases.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures


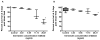

References
-
- Abbott WS. A method of computing the effectiveness of an insecticide. Journal of Economic Entomology. 1925;18:265–276. - PubMed
-
- Baraka OZ, Mahmoud BM, Marschke CK, Geary TG, Homeida MMA, Williams JF. Ivermectin distribution in the plasma and tissues of patients infected with Onchocerca volvulus. European Journal of Clinical Pharmacology. 1996;50:407–410. - PubMed
-
- Beier JC. Frequent blood-feeding and restrictive sugar-feeding behavior enhance the malaria vector potential of Anopheles gambiae s.l. and An. funestus (Diptera:Culicidae) in western Kenya. J Med Entomol. 1996;33:613–8. - PubMed
-
- Black WCI, Moore CG. Population Biology as a Tool to Study Vector-Borne Diseases. In: Marquardt WC, editor. Biology of Disease Vectors. Elsevier Academic Press; San Diego, CA: 2005. pp. 187–206.
-
- Bloomquist JR. Chloride channels as tools for developing selective insecticides. Arch Insect Biochem Physiol. 2003;54:145–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

