Fluorescence-based assays for the assessment of drug interaction with the human transporters OATP1B1 and OATP1B3
- PMID: 20540932
- PMCID: PMC2919811
- DOI: 10.1016/j.ab.2010.06.012
Fluorescence-based assays for the assessment of drug interaction with the human transporters OATP1B1 and OATP1B3
Abstract
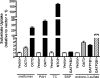
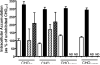
Hepatic disposition plays a significant role in the pharmacokinetics and pharmacodynamics of a variety of drugs. Sinusoidal membrane transporters have been shown to participate in the hepatic disposition of many pharmaceuticals. Two sinusoidal membrane transporters with an established role in hepatic disposition are OATP1B1 and OATP1B3 (organic anion-transporting polypeptides 1B1 and 1B3, respectively). OATP1B1 and OATP1B3 have been implicated in the hepatic uptake of statin drugs, and polymorphisms linked to OATP1B1 have been associated with deleterious patient endpoints. As a result, OATP1B1 and OATP1B3 represent sites for potential drug-drug interactions. Numerous methods exist for identifying potential drug-drug interactions with transporters. However, relatively few offer the convenience and speed of fluorescence-based assays. Here a fluorescence-based assay was developed for measuring the OATP1B1- and OATP1B3-mediated transport of 8-fluorescein-cAMP (8-FcA). The OATP1B1- and OATP1B3-mediated transport of 8-FcA was time dependent and saturable (K(m)=2.9 and 1.8 microM, V(max)=0.20 and 0.33 pmol/min/cm(2), respectively). Molecules known to interact with OATPs, including cyclosporin A, rifampicin, and glibenclamide, each demonstrated concentration-dependent inhibition of 8-FcA transport by OATP1B1 and OATP1B3. The in vitro fluorescence-based assays described here using 8-FcA as the substrate are convenient and rapid and have utility in screening drug candidates for potential drug-drug interactions with OATP1B1 and OATP1B3.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures



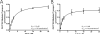
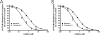
References
-
- Ho RH, Kim RB. Transporters and drug therapy: implications for drug disposition and disease. Clin. Pharmacol. Ther. 2005;78:260–277. - PubMed
-
- Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat. Rev. Drug Discov. 2004;3:711–716. - PubMed
-
- International Transporter Consortium KM, Giacomini SM, Huang DJ, Tweedie LZ, Benet KL, Brouwer X, Chu A, Dahlin R, Evers V, Fischer KM, Hillgren KA, Hoffmaster T, Ishikawa D, Keppler RB, Kim CA, Lee M, Niemi JW, Polli Y, Sugiyama PW, Swaan JA, Ware SH, Wright SW, Yee MJ, Zamek-Gliszczynski L. Zhang, Membrane transporters in drug development. Nat. Rev. Drug. Discov. 2010;9:215–236. - PMC - PubMed
-
- Crespi CL, Stresser DM. Fluorometric screening for metabolism-based drug-drug interactions. J. Pharmacol. Toxicol. Methods. 2000;44:325–331. - PubMed
-
- Ahlin G, Karlsson J, Pedersen JM, Gustavsson L, Larsson R, Matsson P, Norinder U, Bergström CAP. Artursson, Structural requirements for drug inhibition of the liver specific human organic cation transport protein. J. Med. Chem. 2008;51:5932–5942. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

