The remarkable cochlear amplifier
- PMID: 20541061
- PMCID: PMC6366996
- DOI: 10.1016/j.heares.2010.05.001
The remarkable cochlear amplifier
Erratum in
- Hear Res. 2011 Oct;280(1-2):245. Sacchi, J R Santos [corrected to Santos-Sacchi, J]
Abstract
This composite article is intended to give the experts in the field of cochlear mechanics an opportunity to voice their personal opinion on the one mechanism they believe dominates cochlear amplification in mammals. A collection of these ideas are presented here for the auditory community and others interested in the cochlear amplifier. Each expert has given their own personal view on the topic and at the end of their commentary they have suggested several experiments that would be required for the decisive mechanism underlying the cochlear amplifier. These experiments are presently lacking but if successfully performed would have an enormous impact on our understanding of the cochlear amplifier.
Figures
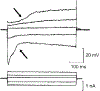

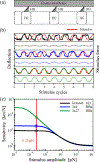

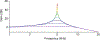
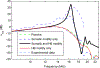
References
-
- Allen JB, 1980. Cochlear micromechanics-a physical model of transduction. J. Acoust. Soc. Am. 68, 1660–1670. - PubMed
-
- Anvari B, Zhang R, Qian F, Rajagopalan L, Pereira FA, Brownell WE, 2007. Effects of prestin on membrane mechanics and electromechanics. Conf. Proc. IEEE Eng. Med. Biol. Soc. 68, 5384–5386. - PubMed
-
- Ashmore J, 2008. Cochlear outer hair cell motility. Physiol. Rev. 88,173–210. - PubMed
-
- Avan P, Bonfils P, Gilain L, Mom T, 2003. Physiopathological significance of distortion-product otoacoustic emissions at 2f1-f2 produced by high-versus low-level stimuli. J. Acoust. Soc. Am. 113, 430–441. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 DC008130/DC/NIDCD NIH HHS/United States
- R01 DC001362/DC/NIDCD NIH HHS/United States
- NIH-NIDCD RO1 DC01362/PHS HHS/International
- NIDCD DC00273/PHS HHS/International
- NIH-NIDCD R01-04084/PHS HHS/International
- F32 DC000354/DC/NIDCD NIH HHS/United States
- R01 DC009913/DC/NIDCD NIH HHS/United States
- R01 DC004084/DC/NIDCD NIH HHS/United States
- R01 DC000273/DC/NIDCD NIH HHS/United States
- DC009913/DC/NIDCD NIH HHS/United States
- R01 DC000089/DC/NIDCD NIH HHS/United States
- R01 DC002775/DC/NIDCD NIH HHS/United States
- DC000354/DC/NIDCD NIH HHS/United States
- DC00089-38/DC/NIDCD NIH HHS/United States
- DC008130/DC/NIDCD NIH HHS/United States
- R01 DC000354/DC/NIDCD NIH HHS/United States
- R01-DC002775/DC/NIDCD NIH HHS/United States
LinkOut - more resources
Full Text Sources


