Clock and cycle limit starvation-induced sleep loss in Drosophila
- PMID: 20541409
- PMCID: PMC2929698
- DOI: 10.1016/j.cub.2010.05.029
Clock and cycle limit starvation-induced sleep loss in Drosophila
Abstract
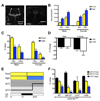
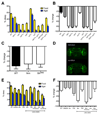
Neural systems controlling the vital functions of sleep and feeding in mammals are tightly interconnected: sleep deprivation promotes feeding, whereas starvation suppresses sleep. Here we show that starvation in Drosophila potently suppresses sleep, suggesting that these two homeostatically regulated behaviors are also integrated in flies. The sleep-suppressing effect of starvation is independent of the mushroom bodies, a previously identified sleep locus in the fly brain, and therefore is regulated by distinct neural circuitry. The circadian clock genes Clock (Clk) and cycle (cyc) are critical for proper sleep suppression during starvation. However, the sleep suppression is independent of light cues and of circadian rhythms as shown by the fact that starved period mutants sleep like wild-type flies. By selectively targeting subpopulations of Clk-expressing neurons, we localize the observed sleep phenotype to the dorsally located circadian neurons. These findings show that Clk and cyc act during starvation to modulate the conflict of whether flies sleep or search for food.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures


References
-
- Horne J. REM sleep, energy balance and 'optimal foraging'. Neurosci Biobehav Rev. 2009;33:466–474. - PubMed
-
- Rechtschaffen A, Bergmann BM. Sleep deprivation in the rat: an update of the 1989 paper. Sleep. 2002;25:18–24. - PubMed
-
- MacFadyen UM, Oswald I, Lewis SA. Starvation and human slow-wave sleep. J Appl Physiol. 1973;35:391–394. - PubMed
-
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

