Comparative metabolism of cyclophosphamide and ifosfamide in the mouse using UPLC-ESI-QTOFMS-based metabolomics
- PMID: 20541539
- PMCID: PMC2941803
- DOI: 10.1016/j.bcp.2010.06.002
Comparative metabolism of cyclophosphamide and ifosfamide in the mouse using UPLC-ESI-QTOFMS-based metabolomics
Abstract
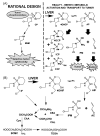
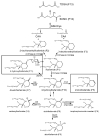
Ifosfamide (IF) and cyclophosphamide (CP) are common chemotherapeutic agents. Interestingly, while the two drugs are isomers, only IF treatment is known to cause nephrotoxicity and neurotoxicity. Therefore, it was anticipated that a comparison of IF and CP drug metabolites in the mouse would reveal reasons for this selective toxicity. Drug metabolites were profiled by ultra-performance liquid chromatography-linked electrospray ionization quadrupole time-of-flight mass spectrometry (UPLC-ESI-QTOFMS), and the results analyzed by multivariate data analysis. Of the total 23 drug metabolites identified by UPLC-ESI-QTOFMS for both IF and CP, five were found to be novel. Ifosfamide preferentially underwent N-dechloroethylation, the pathway yielding 2-chloroacetaldehyde, while cyclophosphamide preferentially underwent ring-opening, the pathway yielding acrolein (AC). Additionally, S-carboxymethylcysteine and thiodiglycolic acid, two downstream IF and CP metabolites, were produced similarly in both IF- and CP-treated mice. This may suggest that other metabolites, perhaps precursors of thiodiglycolic acid, may be responsible for IF encephalopathy and nephropathy.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Figures






Similar articles
-
A comprehensive understanding of thioTEPA metabolism in the mouse using UPLC-ESI-QTOFMS-based metabolomics.Biochem Pharmacol. 2011 Apr 15;81(8):1043-53. doi: 10.1016/j.bcp.2011.01.024. Epub 2011 Feb 12. Biochem Pharmacol. 2011. PMID: 21300029 Free PMC article.
-
New metabolic insights into the mechanism of ifosfamide encephalopathy.Biomed Pharmacother. 2025 Jan;182:117773. doi: 10.1016/j.biopha.2024.117773. Epub 2024 Dec 17. Biomed Pharmacother. 2025. PMID: 39693904
-
Metabolite profiling analysis of hepatitis B virus-induced liver cirrhosis patients with minimal hepatic encephalopathy using gas chromatography-time-of-flight mass spectrometry and ultra-performance liquid chromatography-quadrupole-time-of-flight mass spectrometry.Biomed Chromatogr. 2023 Jan;37(1):e5529. doi: 10.1002/bmc.5529. Epub 2022 Nov 17. Biomed Chromatogr. 2023. PMID: 36250932
-
Neurological toxicity of ifosfamide.Oncology. 2003;65 Suppl 2:11-6. doi: 10.1159/000073352. Oncology. 2003. PMID: 14586141 Review.
-
Ifosfamide - History, efficacy, toxicity and encephalopathy.Pharmacol Ther. 2023 Mar;243:108366. doi: 10.1016/j.pharmthera.2023.108366. Epub 2023 Feb 25. Pharmacol Ther. 2023. PMID: 36842616 Review.
Cited by
-
A comprehensive understanding of thioTEPA metabolism in the mouse using UPLC-ESI-QTOFMS-based metabolomics.Biochem Pharmacol. 2011 Apr 15;81(8):1043-53. doi: 10.1016/j.bcp.2011.01.024. Epub 2011 Feb 12. Biochem Pharmacol. 2011. PMID: 21300029 Free PMC article.
-
Stable isotope- and mass spectrometry-based metabolomics as tools in drug metabolism: a study expanding tempol pharmacology.J Proteome Res. 2013 Mar 1;12(3):1369-76. doi: 10.1021/pr301023x. Epub 2013 Jan 31. J Proteome Res. 2013. PMID: 23301521 Free PMC article.
-
Role of Metabolic Activation in Elemicin-Induced Cellular Toxicity.J Agric Food Chem. 2019 Jul 24;67(29):8243-8252. doi: 10.1021/acs.jafc.9b02137. Epub 2019 Jul 16. J Agric Food Chem. 2019. PMID: 31271289 Free PMC article.
-
Xenobiotic metabolomics: major impact on the metabolome.Annu Rev Pharmacol Toxicol. 2012;52:37-56. doi: 10.1146/annurev-pharmtox-010611-134748. Epub 2011 Aug 3. Annu Rev Pharmacol Toxicol. 2012. PMID: 21819238 Free PMC article. Review.
-
Human metabolites and transformation products of cyclophosphamide and ifosfamide: analysis, occurrence and formation during abiotic treatments.Environ Sci Pollut Res Int. 2016 Jun;23(11):11209-11223. doi: 10.1007/s11356-016-6321-1. Epub 2016 Feb 27. Environ Sci Pollut Res Int. 2016. PMID: 26920534
References
-
- Druckrey H, Raabe S. Specific chemotherapy of carcinoma of the prostate. Klin Wochenschr. 1952;30:882–4. - PubMed
-
- Arnold H, Bourseaux F, Brock N. Chemotherapeutic action of a cyclic nitrogen mustard phosphamide ester (B 518-ASTA) in experimental tumours of the rat. Nature. 1958;181:931. - PubMed
-
- Brock N, Hohorst HJ. Metabolism of cyclophosphamide. Cancer. 1967;20:900–4. - PubMed
-
- Sladek NE. Evidence for an aldehyde possessing alkylating activity as the primary metabolite of cyclophosphamide. Cancer Res. 1973;33:651–8. - PubMed
-
- Hill DL, Laster WR, Jr., Struck RF. Enzymatic metabolism of cyclophosphamide and nicotine and production of a toxic cyclophosphamide metabolite. Cancer Res. 1972;32:658–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

