Recombinant bacterial expression and purification of human fragile X mental retardation protein isoform 1
- PMID: 20541608
- PMCID: PMC2952666
- DOI: 10.1016/j.pep.2010.06.002
Recombinant bacterial expression and purification of human fragile X mental retardation protein isoform 1
Abstract
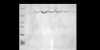
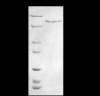
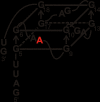
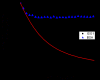
The loss of expression of the fragile X mental retardation protein (FMRP) leads to fragile X syndrome. FMRP has two types of RNA binding domains, two K-homology domains and an arginine-glycine-glycine box domain, and it is proposed to act as a translation regulator of specific messenger RNA. The interest to produce sufficient quantities of pure recombinant FMRP for biochemical and biophysical studies is high. However, the recombinant bacterial expression of FMRP has had limited success, and subsequent recombinant eukaryotic and in vitro expression has also resulted in limited success. In addition, the in vitro and eukaryotic expression systems may produce FMRP which is posttranslationally modified, as phosphorylation and arginine methylation have been shown to occur on FMRP. In this study, we have successfully isolated the conditions for recombinant expression, purification and long-term storage of FMRP using Escherichia coli, with a high yield. The expression of FMRP using E. coli renders the protein devoid of the posttranslational modifications of phosphorylation and arginine methylation, allowing the study of the direct effects of these modifications individually and simultaneously. In order to assure that FMRP retained activity throughout the process, we used fluorescence spectroscopy to assay the binding activity of the FMRP arginine-glycine-glycine box for the semaphorin 3F mRNA and confirmed that FMRP remained active.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Biophysical characterization of G-quadruplex forming FMR1 mRNA and of its interactions with different fragile X mental retardation protein isoforms.RNA. 2014 Jan;20(1):103-14. doi: 10.1261/rna.041442.113. Epub 2013 Nov 18. RNA. 2014. PMID: 24249225 Free PMC article.
-
Analysis of the Fragile X mental retardation protein isoforms 1, 2 and 3 interactions with the G-quadruplex forming semaphorin 3F mRNA.Mol Biosyst. 2012 Feb;8(2):642-9. doi: 10.1039/c1mb05322a. Epub 2011 Dec 1. Mol Biosyst. 2012. PMID: 22134704
-
Expression, identification and purification of human FMRP isoform 10.Front Biosci (Elite Ed). 2012 Jun 1;4(7):2579-83. doi: 10.2741/e567. Front Biosci (Elite Ed). 2012. PMID: 22652662
-
Fragile X mental retardation protein controls ion channel expression and activity.J Physiol. 2016 Oct 15;594(20):5861-5867. doi: 10.1113/JP270675. Epub 2016 May 5. J Physiol. 2016. PMID: 26864773 Free PMC article. Review.
-
Regulating a translational regulator: mechanisms cells use to control the activity of the fragile X mental retardation protein.Cell Mol Life Sci. 2004 Jul;61(14):1714-28. doi: 10.1007/s00018-004-4059-2. Cell Mol Life Sci. 2004. PMID: 15241549 Free PMC article. Review.
Cited by
-
Biophysical characterization of G-quadruplex forming FMR1 mRNA and of its interactions with different fragile X mental retardation protein isoforms.RNA. 2014 Jan;20(1):103-14. doi: 10.1261/rna.041442.113. Epub 2013 Nov 18. RNA. 2014. PMID: 24249225 Free PMC article.
-
FMRP S499 is phosphorylated independent of mTORC1-S6K1 activity.PLoS One. 2014 May 7;9(5):e96956. doi: 10.1371/journal.pone.0096956. eCollection 2014. PLoS One. 2014. PMID: 24806451 Free PMC article.
-
Desmoplakin and talin2 are novel mRNA targets of fragile X-related protein-1 in cardiac muscle.Circ Res. 2011 Jul 22;109(3):262-71. doi: 10.1161/CIRCRESAHA.111.244244. Epub 2011 Jun 9. Circ Res. 2011. PMID: 21659647 Free PMC article.
-
Identification of a molecular locus for normalizing dysregulated GABA release from interneurons in the Fragile X brain.Mol Psychiatry. 2020 Sep;25(9):2017-2035. doi: 10.1038/s41380-018-0240-0. Epub 2018 Sep 17. Mol Psychiatry. 2020. PMID: 30224722 Free PMC article.
-
FMRP - G-quadruplex mRNA - miR-125a interactions: Implications for miR-125a mediated translation regulation of PSD-95 mRNA.PLoS One. 2019 May 21;14(5):e0217275. doi: 10.1371/journal.pone.0217275. eCollection 2019. PLoS One. 2019. PMID: 31112584 Free PMC article.
References
-
- Jin P, Warren ST. Understanding the molecular basis of fragile X syndrome. Human molecular genetics. 2000;9:901–908. - PubMed
-
- O’Donnell WT, Warren ST. A decade of molecular studies of fragile X syndrome. Annual review of neuroscience. 2002;25:315–338. - PubMed
-
- Siomi H, Siomi MC, Nussbaum RL, Dreyfuss G. The protein product of the fragile X gene, FMR1, has characteristics of an RNA-binding protein. Cell. 1993;74:291–298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

