Molecular ultrasound imaging: current status and future directions
- PMID: 20541656
- PMCID: PMC3144865
- DOI: 10.1016/j.crad.2010.02.013
Molecular ultrasound imaging: current status and future directions
Abstract
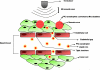
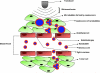
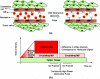
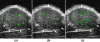
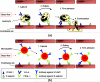
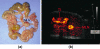
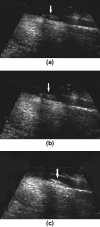
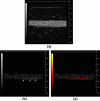
Targeted contrast-enhanced ultrasound (molecular ultrasound) is an emerging imaging strategy that combines ultrasound technology with novel molecularly-targeted ultrasound contrast agents for assessing biological processes at the molecular level. Molecular ultrasound contrast agents are nano- or micro-sized particles that are targeted to specific molecular markers by adding high-affinity binding ligands onto the surface of the particles. Following intravenous administration, these targeted ultrasound contrast agents accumulate at tissue sites overexpressing specific molecular markers, thereby enhancing the ultrasound imaging signal. High spatial and temporal resolution, real-time imaging, non-invasiveness, relatively low costs, lack of ionising irradiation and wide availability of ultrasound systems are advantages compared to other molecular imaging modalities. In this article we review current concepts and future directions of molecular ultrasound imaging, including different classes of molecular ultrasound contrast agents, ongoing technical developments of pre-clinical and clinical ultrasound systems, the potential of molecular ultrasound for imaging different diseases at the molecular level, and the translation of molecular ultrasound into the clinic.
Copyright 2010 The Royal College of Radiologists. Published by Elsevier Ltd. All rights reserved.
Figures

References
-
- Lanza GM, Wickline SA. Targeted ultrasonic contrast agents for molecular imaging and therapy. Curr Probl Cardiol. 2003;28(12):625. - PubMed
-
- Klibanov AL. Molecular imaging with targeted ultrasound contrast microbubbles. Ernst Schering Res Found Workshop. 2005;(49):171. - PubMed
-
- McCulloch M, Gresser C, Moos S, et al. Ultrasound contrast physics: A series on contrast echocardiography, article 3. J Am Soc Echocardiogr. 2000;13(10):959. - PubMed
-
- Klibanov A, Gu H, Wojdyla JK, et al. Attachemnet of ligands to gas filled microbubbles via PEG spacer and lipid residues anchored at the interface. Boston: 1999. p. 124.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

