NSAID sulindac and its analog bind RXRalpha and inhibit RXRalpha-dependent AKT signaling
- PMID: 20541701
- PMCID: PMC2907921
- DOI: 10.1016/j.ccr.2010.04.023
NSAID sulindac and its analog bind RXRalpha and inhibit RXRalpha-dependent AKT signaling
Abstract
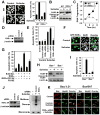
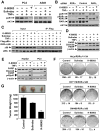
Nonsteroidal anti-inflammatory drugs (NSAIDs) exert their anticancer effects through cyclooxygenase-2 (COX-2)-dependent and independent mechanisms. Here, we report that Sulindac, an NSAID, induces apoptosis by binding to retinoid X receptor-alpha (RXRalpha). We identified an N-terminally truncated RXRalpha (tRXRalpha) in several cancer cell lines and primary tumors, which interacted with the p85alpha subunit of phosphatidylinositol-3-OH kinase (PI3K). Tumor necrosis factor-alpha (TNFalpha) promoted tRXRalpha interaction with the p85alpha, activating PI3K/AKT signaling. When combined with TNFalpha, Sulindac inhibited TNFalpha-induced tRXRalpha/p85alpha interaction, leading to activation of the death receptor-mediated apoptotic pathway. We designed and synthesized a Sulindac analog K-80003, which has increased affinity to RXRalpha but lacks COX inhibitory activity. K-80003 displayed enhanced efficacy in inhibiting tRXRalpha-dependent AKT activation and tRXRalpha tumor growth in animals.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures






Similar articles
-
Sulindac-derived RXRα modulators inhibit cancer cell growth by binding to a novel site.Chem Biol. 2014 May 22;21(5):596-607. doi: 10.1016/j.chembiol.2014.02.017. Epub 2014 Apr 3. Chem Biol. 2014. PMID: 24704507 Free PMC article.
-
Antagonist effect of triptolide on AKT activation by truncated retinoid X receptor-alpha.PLoS One. 2012;7(4):e35722. doi: 10.1371/journal.pone.0035722. Epub 2012 Apr 24. PLoS One. 2012. PMID: 22545132 Free PMC article.
-
Synthesis and SAR study of modulators inhibiting tRXRα-dependent AKT activation.Eur J Med Chem. 2013 Apr;62:632-48. doi: 10.1016/j.ejmech.2013.01.012. Epub 2013 Jan 18. Eur J Med Chem. 2013. PMID: 23434637 Free PMC article.
-
Targeting truncated RXRα for cancer therapy.Acta Biochim Biophys Sin (Shanghai). 2016 Jan;48(1):49-59. doi: 10.1093/abbs/gmv104. Epub 2015 Oct 21. Acta Biochim Biophys Sin (Shanghai). 2016. PMID: 26494413 Free PMC article. Review.
-
Overview of the structure-based non-genomic effects of the nuclear receptor RXRα.Cell Mol Biol Lett. 2018 Aug 7;23:36. doi: 10.1186/s11658-018-0103-3. eCollection 2018. Cell Mol Biol Lett. 2018. PMID: 30093910 Free PMC article. Review.
Cited by
-
Effects of Visceralising Leishmania on the Spleen, Liver, and Bone Marrow: A Pathophysiological Perspective.Microorganisms. 2021 Apr 5;9(4):759. doi: 10.3390/microorganisms9040759. Microorganisms. 2021. PMID: 33916346 Free PMC article. Review.
-
Nitrostyrene Derivatives Act as RXRα Ligands to Inhibit TNFα Activation of NF-κB.Cancer Res. 2015 May 15;75(10):2049-60. doi: 10.1158/0008-5472.CAN-14-2435. Epub 2015 Mar 20. Cancer Res. 2015. PMID: 25795708 Free PMC article.
-
Targeting cancer-related inflammation with non-steroidal anti-inflammatory drugs: Perspectives in pharmacogenomics.Front Pharmacol. 2022 Dec 5;13:1078766. doi: 10.3389/fphar.2022.1078766. eCollection 2022. Front Pharmacol. 2022. PMID: 36545311 Free PMC article. Review.
-
Oncogenic potential of truncated RXRα during colitis-associated colorectal tumorigenesis by promoting IL-6-STAT3 signaling.Nat Commun. 2019 Apr 1;10(1):1463. doi: 10.1038/s41467-019-09375-8. Nat Commun. 2019. PMID: 30931933 Free PMC article.
-
Phase separation of Nur77 mediates celastrol-induced mitophagy by promoting the liquidity of p62/SQSTM1 condensates.Nat Commun. 2021 Oct 13;12(1):5989. doi: 10.1038/s41467-021-26295-8. Nat Commun. 2021. PMID: 34645818 Free PMC article.
References
-
- Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. - PubMed
-
- Altucci L, Gronemeyer H. The promise of retinoids to fight against cancer. Nat Rev Cancer. 2001;1:181–193. - PubMed
-
- Altucci L, Rossin A, Hirsch O, Nebbioso A, Vitoux D, Wilhelm E, Guidez F, De Simone M, Schiavone EM, Grimwade D, et al. Rexinoid-triggered differentiation and tumor-selective apoptosis of acute myeloid leukemia by protein kinase A-mediated desubordination of retinoid X receptor. Cancer Res. 2005;65:8754–8765. - PubMed
-
- Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–371. - PubMed
-
- Bourguet W, Vivat V, Wurtz JM, Chambon P, Gronemeyer H, Moras D. Crystal structure of a heterodimeric complex of RAR and RXR ligand- binding domains. Mol Cell. 2000;5:289–298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

