NSAID sulindac and its analog bind RXRalpha and inhibit RXRalpha-dependent AKT signaling
- PMID: 20541701
- PMCID: PMC2907921
- DOI: 10.1016/j.ccr.2010.04.023
NSAID sulindac and its analog bind RXRalpha and inhibit RXRalpha-dependent AKT signaling
Abstract
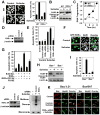
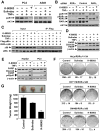
Nonsteroidal anti-inflammatory drugs (NSAIDs) exert their anticancer effects through cyclooxygenase-2 (COX-2)-dependent and independent mechanisms. Here, we report that Sulindac, an NSAID, induces apoptosis by binding to retinoid X receptor-alpha (RXRalpha). We identified an N-terminally truncated RXRalpha (tRXRalpha) in several cancer cell lines and primary tumors, which interacted with the p85alpha subunit of phosphatidylinositol-3-OH kinase (PI3K). Tumor necrosis factor-alpha (TNFalpha) promoted tRXRalpha interaction with the p85alpha, activating PI3K/AKT signaling. When combined with TNFalpha, Sulindac inhibited TNFalpha-induced tRXRalpha/p85alpha interaction, leading to activation of the death receptor-mediated apoptotic pathway. We designed and synthesized a Sulindac analog K-80003, which has increased affinity to RXRalpha but lacks COX inhibitory activity. K-80003 displayed enhanced efficacy in inhibiting tRXRalpha-dependent AKT activation and tRXRalpha tumor growth in animals.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures






References
-
- Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. - PubMed
-
- Altucci L, Gronemeyer H. The promise of retinoids to fight against cancer. Nat Rev Cancer. 2001;1:181–193. - PubMed
-
- Altucci L, Rossin A, Hirsch O, Nebbioso A, Vitoux D, Wilhelm E, Guidez F, De Simone M, Schiavone EM, Grimwade D, et al. Rexinoid-triggered differentiation and tumor-selective apoptosis of acute myeloid leukemia by protein kinase A-mediated desubordination of retinoid X receptor. Cancer Res. 2005;65:8754–8765. - PubMed
-
- Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–371. - PubMed
-
- Bourguet W, Vivat V, Wurtz JM, Chambon P, Gronemeyer H, Moras D. Crystal structure of a heterodimeric complex of RAR and RXR ligand- binding domains. Mol Cell. 2000;5:289–298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

