Differential expression of the p65 gene family
- PMID: 2054189
- PMCID: PMC4704688
- DOI: 10.1016/0896-6273(91)90239-v
Differential expression of the p65 gene family
Abstract
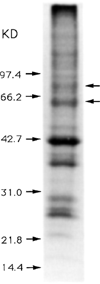
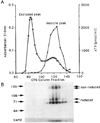
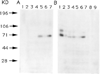
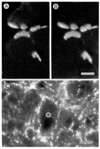
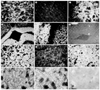
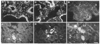
The genome of the marine ray Discopyge ommata contains at least three p65-related genes. o-p65-A is 84% identical, o-p65-B is 78% identical, and o-p65-C is only 41% identical to a previously characterized rat p65. The cytoplasmic domain, particularly the two regions that are similar to the regulatory domain of protein kinase C, are most highly conserved. The three genes are expressed in different but overlapping patterns in the central nervous system. o-p65-A immunoreactivity is found predominantly in forebrain, cerebellum, and neuroendocrine cells, while o-p65-B immunoreactivity is predominantly localized to the spinal cord, brainstem, and midbrain. Many synaptic vesicle proteins are members of small gene families that are differentially expressed, resulting in several unique combinations of these molecules in specific brain regions.
Figures






References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

