Constitutively active Lck kinase in T cells drives antigen receptor signal transduction
- PMID: 20541955
- PMCID: PMC2996607
- DOI: 10.1016/j.immuni.2010.05.011
Constitutively active Lck kinase in T cells drives antigen receptor signal transduction
Abstract
T cell antigen receptor (TCR) and coreceptor ligation is thought to initiate signal transduction by inducing activation of the kinase Lck. Here we showed that catalytically active Lck was present in unstimulated naive T cells and thymocytes and was readily detectable in these cells in lymphoid organs. In naive T cells up to approximately 40% of total Lck was constitutively activated, part of which was also phosphorylated on the C-terminal inhibitory site. Formation of activated Lck was independent of TCR and coreceptors but required Lck catalytic activity and its maintenance relied on monitoring by the HSP90-CDC37 chaperone complex to avoid degradation. The amount of activated Lck did not change after TCR and coreceptor engagement; however it determined the extent of TCR-zeta phosphorylation. Our findings suggest a dynamic regulation of Lck activity that can be promptly utilized to initiate T cell activation and have implications for signaling by other immune receptors.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures
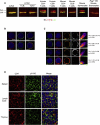
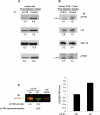

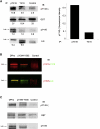
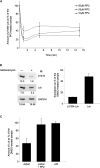
References
-
- Acuto O., Di Bartolo V., Michel F. Tailoring T-cell receptor signals by proximal negative feedback mechanisms. Nat. Rev. Immunol. 2008;8:699–712. - PubMed
-
- Au-Yeung B.B., Deindl S., Hsu L.Y., Palacios E.H., Levin S.E., Kuriyan J., Weiss A. The structure, regulation, and function of ZAP-70. Immunol. Rev. 2009;228:41–57. - PubMed
-
- Baker B.M., Wiley D.C. alpha beta T cell receptor ligand-specific oligomerization revisited. Immunity. 2001;14:681–692. - PubMed
-
- Beddoe T., Chen Z., Clements C.S., Ely L.K., Bushell S.R., Vivian J.P., Kjer-Nielsen L., Pang S.S., Dunstone M.A., Liu Y.C. Antigen ligation triggers a conformational change within the constant domain of the alphabeta T cell receptor. Immunity. 2009;30:777–788. - PubMed
-
- Boggon T.J., Eck M.J. Structure and regulation of Src family kinases. Oncogene. 2004;23:7918–7927. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

