The ISG15 conjugation system broadly targets newly synthesized proteins: implications for the antiviral function of ISG15
- PMID: 20542004
- PMCID: PMC2887317
- DOI: 10.1016/j.molcel.2010.05.002
The ISG15 conjugation system broadly targets newly synthesized proteins: implications for the antiviral function of ISG15
Abstract
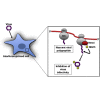
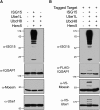
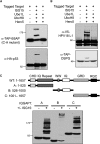
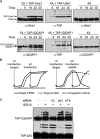
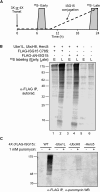
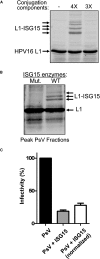
ISG15 is an interferon-induced and antiviral ubiquitin-like protein (Ubl). Herc5, the major E3 enzyme for ISG15, mediates the ISGylation of more than 300 proteins in interferon-stimulated cells. In addressing this broad substrate selectivity of Herc5, we found that: (1) the range of substrates extends even further and includes many exogenously expressed foreign proteins, (2) ISG15 conjugation is restricted to newly synthesized pools of proteins, and (3) Herc5 is physically associated with polyribosomes. These results lead to a model for ISGylation in which Herc5 broadly modifies newly synthesized proteins in a cotranslational manner. This further suggests that, in the context of an interferon-stimulated cell, newly translated viral proteins may be primary targets of ISG15. Consistent with this, we demonstrate that ISGylation of human papillomavirus (HPV) L1 capsid protein has a dominant-inhibitory effect on the infectivity of HPV16 pseudoviruses.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

