Monoubiquitination of RPN10 regulates substrate recruitment to the proteasome
- PMID: 20542005
- PMCID: PMC3282119
- DOI: 10.1016/j.molcel.2010.05.001
Monoubiquitination of RPN10 regulates substrate recruitment to the proteasome
Abstract
The proteasome recognizes its substrates via a diverse set of ubiquitin receptors, including subunits Rpn10/S5a and Rpn13. In addition, shuttling factors, such as Rad23, recruit substrates to the proteasome by delivering ubiquitinated proteins. Despite the increasing understanding of the factors involved in this process, the regulation of substrate delivery remains largely unexplored. Here we report that Rpn10 is monoubiquitinated in vivo and that this modification has profound effects on proteasome function. Monoubiquitination regulates the capacity of Rpn10 to interact with substrates by inhibiting Rpn10's ubiquitin-interacting motif (UIM). We show that Rsp5, a member of NEDD4 ubiquitin-protein ligase family, and Ubp2, a deubiquitinating enzyme, control the levels of Rpn10 monoubiquitination in vivo. Notably, monoubiquitination of Rpn10 is decreased under stress conditions, suggesting a mechanism of control of receptor availability mediated by the Rsp5-Ubp2 system. Our results reveal an unanticipated link between monoubiquitination signal and regulation of proteasome function.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Figures


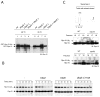
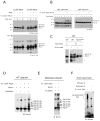



Comment in
-
Regulating the regulator: Rsp5 ubiquitinates the proteasome.Mol Cell. 2010 Jun 11;38(5):623-4. doi: 10.1016/j.molcel.2010.05.029. Mol Cell. 2010. PMID: 20541994
References
-
- Ciechanover A, Brundin P. The ubiquitin proteasome system in neurodegenerative diseases: sometimes the chicken, sometimes the egg. Neuron. 2003;40:427–446. - PubMed
-
- Crosas B, Hanna J, Kirkpatrick DS, Zhang DP, Tone Y, Hathaway NA, Buecker C, Leggett DS, Schmidt M, King RW, Gygi SP, Finley D. Ubiquitin chains are remodeled at the proteasome by opposing ubiquitin ligase and deubiquitinating activities. Cell. 2006;127:1401–1413. - PubMed
-
- Deveraux Q, Ustrell V, Pickart C, Rechsteiner M. A 26 S protease subunit that binds ubiquitin conjugates. J Biol Chem. 1994;269:7059–7061. - PubMed
-
- Di Fiore PP, Polo S, Hofmann K. When ubiquitin meets ubiquitin receptors: a signalling connection. Nat Rev Mol Cell Biol. 2003;4:491–497. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

