Identification of key residues involved in fibril formation by the conserved N-terminal region of Plasmodium falciparum merozoite surface protein 2 (MSP2)
- PMID: 20542076
- PMCID: PMC2948610
- DOI: 10.1016/j.biochi.2010.06.001
Identification of key residues involved in fibril formation by the conserved N-terminal region of Plasmodium falciparum merozoite surface protein 2 (MSP2)
Abstract
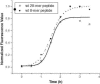
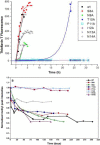
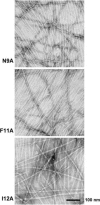
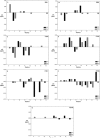
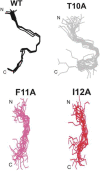
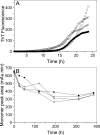
Merozoite surface protein 2 (MSP2) from the human malaria parasite Plasmodium falciparum is expressed as a GPI-anchored protein on the merozoite surface. MSP2 is assumed to have a role in erythrocyte invasion and is a leading vaccine candidate. Recombinant MSP2 forms amyloid-like fibrils upon storage, as do peptides corresponding to sequences in the conserved N-terminal region, which constitutes the structural core of fibrils formed by full-length MSP2. We have investigated the roles of individual residues in fibril formation and local ordered structure in two peptides, a recombinant 25-residue peptide corresponding to the entire N-terminal domain of mature MSP2 and an 8-residue peptide from the central region of this domain (residues 8-15). Both peptides formed fibrils that were similar to amyloid-like fibrils formed by full-length MSP2. Phe11 and Ile12 have important roles both in stabilising local structure in these peptides and promoting fibril formation; the F11A and I12A mutants of MSP2(8-15) were essentially unstructured in solution and fibril formation at pH 7.4 and 4.7 was markedly retarded. The T10A mutant showed intermediate behaviour, having a less well defined structure than wild-type and slower fibril formation at pH 7.4. The mutation of Phe11 and Ile12 in MSP2(1-25) significantly retarded but did not abolish fibril formation, indicating that these residues also play a key role in fibril formation by the entire N-terminal conserved region. These mutations had little effect on the aggregation of full-length MSP2, however, suggesting that regions outside the conserved N-terminus have unanticipated importance for fibril formation in the full-length protein.
Copyright © 2010 Elsevier Masson SAS. All rights reserved.
Figures



References
-
- Greenwood B. Between hope and a hard place. Nature. 2004;430:926–927. - PubMed
-
- Aponte JJ, Aide P, Renom M, Mandomando I, Bassat Q, Sacarlal J, Manaca MN, Lafuente S, Barbosa A, Leach A, Lievens M, Vekemans J, Sigauque B, Dubois MC, Demoitie MA, Sillman M, Savarese B, McNeil JG, Macete E, Ballou WR, Cohen J, Alonso PL. Safety of the RTS,S/AS02D candidate malaria vaccine in infants living in a highly endemic area of Mozambique: a double blind randomised controlled phase I/IIb trial. Lancet. 2007;370:1543–1551. - PubMed
-
- Anders RF, Adda CG, Foley M, Norton RS. Recombinant protein vaccines against the asexual blood-stages of Plasmodium falciparum. Human Vaccines. 2010;6:39–53. - PubMed
-
- Genton B, Betuela I, Felger I, Al-Yaman F, Anders RF, Saul A, Rare L, Baisor M, Lorry K, Brown GV, Pye D, Irving DO, Smith TA, Beck HP, Alpers MP. A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1-2b trial in Papua New Guinea. J Infect Dis. 2002;185:820–827. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

