Ventilatory impairment in the dysmyelinated Long Evans shaker rat
- PMID: 20542092
- PMCID: PMC2927872
- DOI: 10.1016/j.neuroscience.2010.06.010
Ventilatory impairment in the dysmyelinated Long Evans shaker rat
Abstract
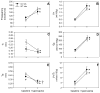
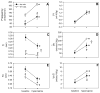
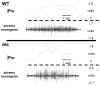
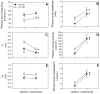
Although respiratory complications significantly contribute to morbidity/mortality in advanced myelin disorders, little is known concerning mechanisms whereby dysmyelination impairs ventilation, or how patients compensate (i.e. plasticity). To establish a model for studies concerning mechanisms of ventilatory impairment/compensation, we tested the hypotheses that respiratory function progressively declines in a model of CNS dysmyelination, the Long Evans shaker rat (les). The observed impairment is associated with abnormal inspiratory neural output. Minimal myelin staining was found throughout the CNS of les rats, including the brainstem and cervical bulbospinal tracts. Ventilation (via whole-body plethysmography) and phrenic motor output were assessed in les and wild-type (WT) rats during baseline, hypoxia (11% O(2)) and hypercapnia (7% CO(2)). Hypercapnic ventilatory responses were similar in young adult les and WT rats (2 months old); in hypoxia, rats exhibited seizure-like activity with sustained apneas. However, 5-6 month old les rats exhibited decreased breathing frequencies, mean inspiratory flow (V(T)/T(I)) and ventilation (V (E)) during baseline and hypercapnia. Although phrenic motor output exhibited normal burst frequency and amplitude in 5-6 month old les rats, intra-burst activity was abnormal. In WT rats, phrenic activity was progressive and augmenting; in les rats, phrenic activity was decrementing with asynchronized, multipeaked activity. Thus, although ventilatory capacity is maintained in young, dysmyelinated rats, ventilatory impairment develops with age, possibly through discoordination in respiratory motor output. This study is the first reporting age-related breathing abnormalities in a rodent dysmyelination model, and provides the foundation for mechanistic studies of respiratory insufficiency and therapeutic interventions.
Copyright (c) 2010 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Cervical spinal demyelination with ethidium bromide impairs respiratory (phrenic) activity and forelimb motor behavior in rats.Neuroscience. 2013 Jan 15;229:77-87. doi: 10.1016/j.neuroscience.2012.10.066. Epub 2012 Nov 14. Neuroscience. 2013. PMID: 23159317 Free PMC article.
-
Structural and functional alterations of spinal cord axons in adult Long Evans Shaker (LES) dysmyelinated rats.Exp Neurol. 2005 Jun;193(2):334-49. doi: 10.1016/j.expneurol.2005.01.019. Exp Neurol. 2005. PMID: 15869936
-
Cancer cachexia impairs neural respiratory drive in hypoxia but not hypercapnia.J Cachexia Sarcopenia Muscle. 2019 Feb;10(1):63-72. doi: 10.1002/jcsm.12348. Epub 2018 Oct 25. J Cachexia Sarcopenia Muscle. 2019. PMID: 30362273 Free PMC article.
-
Morphological and morphometric studies of the dysmyelinating mutant, the Long Evans shaker rat.J Neurocytol. 1998 Aug;27(8):581-91. doi: 10.1023/a:1006922227791. J Neurocytol. 1998. PMID: 10405025
-
Neuropathology of bouncer Long Evans, a novel dysmyelinated rat.Comp Med. 2000 Oct;50(5):503-10. Comp Med. 2000. PMID: 11099133
Cited by
-
Common mechanisms of compensatory respiratory plasticity in spinal neurological disorders.Respir Physiol Neurobiol. 2013 Nov 1;189(2):419-28. doi: 10.1016/j.resp.2013.05.025. Epub 2013 May 28. Respir Physiol Neurobiol. 2013. PMID: 23727226 Free PMC article. Review.
-
Cervical spinal demyelination with ethidium bromide impairs respiratory (phrenic) activity and forelimb motor behavior in rats.Neuroscience. 2013 Jan 15;229:77-87. doi: 10.1016/j.neuroscience.2012.10.066. Epub 2012 Nov 14. Neuroscience. 2013. PMID: 23159317 Free PMC article.
-
Changes to Ventilation, Vocalization, and Thermal Nociception in the Pink1-/- Rat Model of Parkinson's Disease.J Parkinsons Dis. 2020;10(2):489-504. doi: 10.3233/JPD-191853. J Parkinsons Dis. 2020. PMID: 32065805 Free PMC article.
References
-
- Behan M, Zabka AG, Thomas CF, Mitchell GS. Sex steroid hormones and the control of breathing. Respir Physiol Neurobiol. 2003;136(2–3):249–263. - PubMed
-
- Buyse B, Demedts M, Meekers J, Vandegaer L, Rochette F, Kerkhofs L. Respiratory dysfunction in multiple sclerosis: a prospective analysis of 60 patients. Eur Respir J. 1997;10:139–145. - PubMed
-
- Carter JL, Noseworthy JH. Ventilatory dysfunction in multiple sclerosis. Clin Chest Med. 1994;15(4):693–703. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

