Human formyl peptide receptor 2 senses highly pathogenic Staphylococcus aureus
- PMID: 20542250
- PMCID: PMC3417054
- DOI: 10.1016/j.chom.2010.05.012
Human formyl peptide receptor 2 senses highly pathogenic Staphylococcus aureus
Abstract
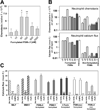
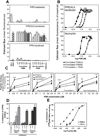
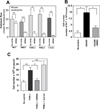
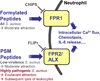
Virulence of emerging community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) and other highly pathogenic S. aureus strains depends on their production of phenol-soluble modulin (PSM) peptide toxins, which combine the capacities to attract and lyse neutrophils. The molecular basis of PSM-stimulated neutrophil recruitment has remained unclear. Here, we demonstrate that the human formyl peptide receptor 2 (FPR2/ALX), which has previously been implicated in control of endogenous inflammatory processes, senses PSMs at nanomolar concentrations and initiates proinflammatory neutrophil responses to CA-MRSA. Specific blocking of FPR2/ALX or deletion of PSM genes in CA-MRSA severely diminished neutrophil detection of CA-MRSA. Furthermore, a specific inhibitor of FPR2/ALX and of its functional mouse counterpart blocked PSM-mediated leukocyte infiltration in vivo in a mouse model. Thus, the innate immune system uses a distinct FPR2/ALX-dependent mechanism to specifically sense bacterial peptide toxins and detect highly virulent bacterial pathogens. FPR2/ALX represents an attractive target for new anti-infective or anti-inflammatory strategies.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Figures

Comment in
-
Phenol-soluble modulins of Staphylococcus aureus lure neutrophils into battle.Cell Host Microbe. 2010 Jun 25;7(6):423-4. doi: 10.1016/j.chom.2010.05.015. Cell Host Microbe. 2010. PMID: 20542245
References
-
- Alvarez V, Coto E, Setien F, Gonzalez-Roces S, Lopez-Larrea C. Molecular evolution of the N-formyl peptide and C5a receptors in non-human primates. Immunogenetics. 1996;44:446–452. - PubMed
-
- Bubeck WJ, Bae T, Otto M, DeLeo FR, Schneewind O. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat. Med. 2007;13:1405–1406. - PubMed
-
- Christophe T, Karlsson A, Dugave C, Rabiet MJ, Boulay F, Dahlgren C. The synthetic peptide Trp-Lys-Tyr-Met-Val-Met-NH2 specifically activates neutrophils through FPRL1/lipoxin A4 receptors and is an agonist for the orphan monocyte-expressed chemoattractant receptor FPRL2. J. Biol. Chem. 2001;276:21585–21593. - PubMed
-
- Cui Y, Le Y, Yazawa H, Gong W, Wang JM. Potential role of the formyl peptide receptor-like 1 (FPRL1) in inflammatory aspects of Alzheimer's disease. J. Leukoc. Biol. 2002;72:628–635. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

