Modeling the role of the coronary vasculature during external field stimulation
- PMID: 20542762
- PMCID: PMC2976591
- DOI: 10.1109/TBME.2010.2051227
Modeling the role of the coronary vasculature during external field stimulation
Abstract
The exact mechanisms by which defibrillation shocks excite cardiac tissue far from both the electrodes and heart surfaces require elucidation. Bidomain theory explains this phenomena through the existence of intramural virtual electrodes (VEs), caused by discontinuities in myocardial tissue structure. In this study, we assess the modeling components essential in constructing a finite-element cardiac tissue model including blood vessels from high-resolution magnetic resonance data and investigate the specific role played by coronary vasculature in VE formation, which currently remains largely unknown. We use a novel method for assigning histologically based fiber architecture around intramural structures and include an experimentally derived vessel lumen wall conductance within the model. Shock-tissue interaction in the presence of vessels is assessed through comparison with a simplified model lacking intramural structures. Results indicate that VEs form around blood vessels for shocks > 8 V/cm. The magnitude of induced polarizations is attenuated by realistic representation of fiber negotiation around vessel cavities, as well as the insulating effects of the vessel lumen wall. Furthermore, VEs formed around large subepicardial vessels reduce epicardial polarization levels. In conclusion, we have found that coronary vasculature acts as an important substrate for VE formation, which may help interpretation of optical mapping data.
Figures



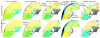




Similar articles
-
Bidomain Predictions of Virtual Electrode-Induced Make and Break Excitations around Blood Vessels.Front Bioeng Biotechnol. 2017 Mar 27;5:18. doi: 10.3389/fbioe.2017.00018. eCollection 2017. Front Bioeng Biotechnol. 2017. PMID: 28396856 Free PMC article.
-
Investigating the role of the coronary vasculature in the mechanisms of defibrillation.Circ Arrhythm Electrophysiol. 2012 Feb;5(1):210-9. doi: 10.1161/CIRCEP.111.965095. Epub 2011 Dec 8. Circ Arrhythm Electrophysiol. 2012. PMID: 22157522 Free PMC article.
-
Evaluating intramural virtual electrodes in the myocardial wedge preparation: simulations of experimental conditions.Biophys J. 2008 Mar 1;94(5):1904-15. doi: 10.1529/biophysj.107.121343. Epub 2007 Nov 9. Biophys J. 2008. PMID: 17993491 Free PMC article.
-
Mechanisms for electrical stimulation of excitable tissue.Crit Rev Biomed Eng. 1994;22(3-4):253-305. Crit Rev Biomed Eng. 1994. PMID: 8598130 Review.
-
Mechanisms of defibrillation.Annu Rev Biomed Eng. 2010 Aug 15;12:233-58. doi: 10.1146/annurev-bioeng-070909-105305. Annu Rev Biomed Eng. 2010. PMID: 20450352 Free PMC article. Review.
Cited by
-
Diastolic field stimulation: the role of shock duration in epicardial activation and propagation.Biophys J. 2013 Jul 16;105(2):523-32. doi: 10.1016/j.bpj.2013.06.009. Biophys J. 2013. PMID: 23870273 Free PMC article.
-
Virtual electrodes around anatomical structures and their roles in defibrillation.PLoS One. 2017 Mar 2;12(3):e0173324. doi: 10.1371/journal.pone.0173324. eCollection 2017. PLoS One. 2017. PMID: 28253365 Free PMC article.
-
Arrhythmia risk stratification of patients after myocardial infarction using personalized heart models.Nat Commun. 2016 May 10;7:11437. doi: 10.1038/ncomms11437. Nat Commun. 2016. PMID: 27164184 Free PMC article.
-
Bidomain Predictions of Virtual Electrode-Induced Make and Break Excitations around Blood Vessels.Front Bioeng Biotechnol. 2017 Mar 27;5:18. doi: 10.3389/fbioe.2017.00018. eCollection 2017. Front Bioeng Biotechnol. 2017. PMID: 28396856 Free PMC article.
-
Efficient simulation of cardiac electrical propagation using high order finite elements.J Comput Phys. 2012 May 20;231(10):3946-3962. doi: 10.1016/j.jcp.2012.01.037. J Comput Phys. 2012. PMID: 24976644 Free PMC article.
References
-
- Zipes D, Fischer J, King R, Nicoll A, Jolly W. Termination of ventricular fibrillation in dogs by depolarizing a critical amount of myocardium. Am J Cardiol. 1975;36:37–44. - PubMed
-
- Zhou X, Daubert J, Wolf P, Smith W, Ideker R. Epicardial mapping of ventricular defibrillation with monophasic and biphasic shocks in dogs. Circ Res. 1993;72:145–160. - PubMed
-
- Fishler MG. Syncytial heterogeneity as a mechanism underlying cardiac far-field stimulation during defibrillation-level shocks. J Cardiovasc Electrophysiol. 1998;9:384–94. - PubMed
-
- Newton JC, Knisley SB, Zhou X, Pollard AE, Ideker RE. Review of mechanisms by which electrical stimulation alters the transmembrane potential. J Cardiovasc Electrophysiol. 1999;10:234–43. - PubMed

