Redirecting reductant flux into hydrogen production via metabolic engineering of fermentative carbon metabolism in a cyanobacterium
- PMID: 20543051
- PMCID: PMC2916493
- DOI: 10.1128/AEM.00862-10
Redirecting reductant flux into hydrogen production via metabolic engineering of fermentative carbon metabolism in a cyanobacterium
Abstract
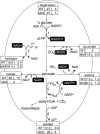
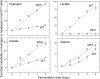
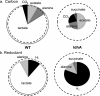
Some aquatic microbial oxygenic photoautotrophs (AMOPs) make hydrogen (H(2)), a carbon-neutral, renewable product derived from water, in low yields during autofermentation (anaerobic metabolism) of intracellular carbohydrates previously stored during aerobic photosynthesis. We have constructed a mutant (the ldhA mutant) of the cyanobacterium Synechococcus sp. strain PCC 7002 lacking the enzyme for the NADH-dependent reduction of pyruvate to D-lactate, the major fermentative reductant sink in this AMOP. Both nuclear magnetic resonance (NMR) spectroscopy and liquid chromatography-mass spectrometry (LC-MS) metabolomic methods have shown that autofermentation by the ldhA mutant resulted in no D-lactate production and higher concentrations of excreted acetate, alanine, succinate, and hydrogen (up to 5-fold) compared to that by the wild type. The measured intracellular NAD(P)(H) concentrations demonstrated that the NAD(P)H/NAD(P)(+) ratio increased appreciably during autofermentation in the ldhA strain; we propose this to be the principal source of the observed increase in H(2) production via an NADH-dependent, bidirectional [NiFe] hydrogenase. Despite the elevated NAD(P)H/NAD(P)(+) ratio, no decrease was found in the rate of anaerobic conversion of stored carbohydrates. The measured energy conversion efficiency (ECE) from biomass (as glucose equivalents) converted to hydrogen in the ldhA mutant is 12%. Together with the unimpaired photoautotrophic growth of the ldhA mutant, these attributes reveal that metabolic engineering is an effective strategy to enhance H(2) production in AMOPs without compromising viability.
Figures

Similar articles
-
Consequences of ccmR deletion on respiration, fermentation and H2 metabolism in cyanobacterium Synechococcus sp. PCC 7002.Biotechnol Bioeng. 2016 Jul;113(7):1448-59. doi: 10.1002/bit.25913. Epub 2016 Jan 28. Biotechnol Bioeng. 2016. PMID: 26704377
-
Synechococcus sp. strain PCC 7002 nifJ mutant lacking pyruvate:ferredoxin oxidoreductase.Appl Environ Microbiol. 2011 Apr;77(7):2435-44. doi: 10.1128/AEM.02792-10. Epub 2011 Feb 11. Appl Environ Microbiol. 2011. PMID: 21317262 Free PMC article.
-
Inactivation of nitrate reductase alters metabolic branching of carbohydrate fermentation in the cyanobacterium Synechococcus sp. strain PCC 7002.Biotechnol Bioeng. 2016 May;113(5):979-88. doi: 10.1002/bit.25862. Epub 2015 Nov 2. Biotechnol Bioeng. 2016. PMID: 26479976
-
Glucose and lactate metabolism by Actinomyces naeslundii.Crit Rev Oral Biol Med. 1999;10(4):487-503. doi: 10.1177/10454411990100040501. Crit Rev Oral Biol Med. 1999. PMID: 10634585 Review.
-
Metabolically engineered bacteria for producing hydrogen via fermentation.Microb Biotechnol. 2008 Mar;1(2):107-25. doi: 10.1111/j.1751-7915.2007.00009.x. Microb Biotechnol. 2008. PMID: 21261829 Free PMC article. Review.
Cited by
-
Synechococcus elongatus UTEX 2973, a fast growing cyanobacterial chassis for biosynthesis using light and CO₂.Sci Rep. 2015 Jan 30;5:8132. doi: 10.1038/srep08132. Sci Rep. 2015. PMID: 25633131 Free PMC article.
-
Functional genomics of novel secondary metabolites from diverse cyanobacteria using untargeted metabolomics.Mar Drugs. 2013 Sep 30;11(10):3617-31. doi: 10.3390/md11103617. Mar Drugs. 2013. PMID: 24084783 Free PMC article.
-
Emerging Trends in Genetic Engineering of Microalgae for Commercial Applications.Mar Drugs. 2022 Apr 24;20(5):285. doi: 10.3390/md20050285. Mar Drugs. 2022. PMID: 35621936 Free PMC article. Review.
-
Cyanobacteria: Photoautotrophic Microbial Factories for the Sustainable Synthesis of Industrial Products.Biomed Res Int. 2015;2015:754934. doi: 10.1155/2015/754934. Epub 2015 Jun 25. Biomed Res Int. 2015. PMID: 26199945 Free PMC article. Review.
-
Cyanobacteria as a Promising Alternative for Sustainable Environment: Synthesis of Biofuel and Biodegradable Plastics.Front Microbiol. 2022 Jul 13;13:939347. doi: 10.3389/fmicb.2022.939347. eCollection 2022. Front Microbiol. 2022. PMID: 35903468 Free PMC article. Review.
References
-
- Becker, A., M. Schmidt, W. Jäger, and A. Pühler. 1995. New gentamicin-resistance and lacZ promoter-probe cassettes suitable for insertion mutagenesis and generation of transcriptional fusions. Gene 162:37-39. - PubMed
-
- Carrieri, D., G. Ananyev, A. M. Garcia Costas, D. A. Bryant, and G. C. Dismukes. 2008. Renewable hydrogen production by cyanobacteria: nickel requirements for optimal hydrogenase activity. Int. J. Hydrogen Energy 33:2014-2022.
-
- Carrieri, D., K. McNeely, A. C. DeRoo, N. Bennette, I. Pelczer, and G. C. Dismukes. 2009. Identification and quantification of water-soluble metabolites by cryoprobe-assisted nuclear magnetic resonance spectroscopy applied to microbial fermentation. Magn. Reson. Chem. 47:S138-S146. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

