Nuclear dynamics during germination, conidiation, and hyphal fusion of Fusarium oxysporum
- PMID: 20543061
- PMCID: PMC2918926
- DOI: 10.1128/EC.00040-10
Nuclear dynamics during germination, conidiation, and hyphal fusion of Fusarium oxysporum
Abstract
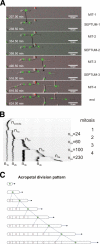
In many fungal pathogens, infection is initiated by conidial germination. Subsequent stages involve germ tube elongation, conidiation, and vegetative hyphal fusion (anastomosis). Here, we used live-cell fluorescence to study the dynamics of green fluorescent protein (GFP)- and cherry fluorescent protein (ChFP)-labeled nuclei in the plant pathogen Fusarium oxysporum. Hyphae of F. oxysporum have uninucleated cells and exhibit an acropetal nuclear pedigree, where only the nucleus in the apical compartment is mitotically active. In contrast, conidiation follows a basopetal pattern, whereby mononucleated microconidia are generated by repeated mitotic cycles of the subapical nucleus in the phialide, followed by septation and cell abscission. Vegetative hyphal fusion is preceded by directed growth of the fusion hypha toward the receptor hypha and followed by a series of postfusion nuclear events, including mitosis of the apical nucleus of the fusion hypha, migration of a daughter nucleus into the receptor hypha, and degradation of the resident nucleus. These previously unreported patterns of nuclear dynamics in F. oxysporum could be intimately related to its pathogenic lifestyle.
Figures





Similar articles
-
Autophagy contributes to regulation of nuclear dynamics during vegetative growth and hyphal fusion in Fusarium oxysporum.Autophagy. 2015;11(1):131-44. doi: 10.4161/15548627.2014.994413. Autophagy. 2015. PMID: 25560310 Free PMC article.
-
Dynamics of the establishment of multinucleate compartments in Fusarium oxysporum.Eukaryot Cell. 2015 Jan;14(1):78-85. doi: 10.1128/EC.00200-14. Epub 2014 Nov 14. Eukaryot Cell. 2015. PMID: 25398376 Free PMC article.
-
Biocontrol strain Pseudomonas fluorescens WCS365 inhibits germination of Fusarium oxysporum spores in tomato root exudate as well as subsequent formation of new spores.Environ Microbiol. 2008 Sep;10(9):2455-61. doi: 10.1111/j.1462-2920.2008.01638.x. Epub 2008 Apr 23. Environ Microbiol. 2008. PMID: 18430156
-
Fusarium oxysporum f. sp. lycopersici C2H2 transcription factor FolCzf1 is required for conidiation, fusaric acid production, and early host infection.Curr Genet. 2019 Jun;65(3):773-783. doi: 10.1007/s00294-019-00931-9. Epub 2019 Jan 10. Curr Genet. 2019. PMID: 30631890
-
FoSTUA, encoding a basic helix-loop-helix protein, differentially regulates development of three kinds of asexual spores, macroconidia, microconidia, and chlamydospores, in the fungal plant pathogen Fusarium oxysporum.Eukaryot Cell. 2004 Dec;3(6):1412-22. doi: 10.1128/EC.3.6.1412-1422.2004. Eukaryot Cell. 2004. PMID: 15590816 Free PMC article.
Cited by
-
Autophagy contributes to regulation of nuclear dynamics during vegetative growth and hyphal fusion in Fusarium oxysporum.Autophagy. 2015;11(1):131-44. doi: 10.4161/15548627.2014.994413. Autophagy. 2015. PMID: 25560310 Free PMC article.
-
A conserved homeobox transcription factor Htf1 is required for phialide development and conidiogenesis in Fusarium species.PLoS One. 2012;7(9):e45432. doi: 10.1371/journal.pone.0045432. Epub 2012 Sep 21. PLoS One. 2012. PMID: 23029006 Free PMC article.
-
Expansion Microscopy for Cell Biology Analysis in Fungi.Front Microbiol. 2020 Apr 3;11:574. doi: 10.3389/fmicb.2020.00574. eCollection 2020. Front Microbiol. 2020. PMID: 32318047 Free PMC article.
-
Role of Autophagy-Related Gene atg22 in Developmental Process and Virulence of Fusarium oxysporum.Genes (Basel). 2019 May 13;10(5):365. doi: 10.3390/genes10050365. Genes (Basel). 2019. PMID: 31086099 Free PMC article.
-
Dynamics of the establishment of multinucleate compartments in Fusarium oxysporum.Eukaryot Cell. 2015 Jan;14(1):78-85. doi: 10.1128/EC.00200-14. Epub 2014 Nov 14. Eukaryot Cell. 2015. PMID: 25398376 Free PMC article.
References
-
- Aylmore R. C., Todd N. K. 1984. Hyphal fusion in Coriolus versicolor, p. 103–125 InJennings D. H., Rayner A. D. M. (ed.), The ecology and physiology of the fungal mycelium. Symposium of the British Mycological Society. Cambridge University Press, Cambridge, United Kingdom
-
- Barhoom S., Sharon A. 2004. cAMP regulation of “pathogenic” and “saprophytic” fungal spore germination. Fungal Genet. Biol. 41:317–326 - PubMed
-
- Bensaude M. 1918. Récherches sur le cycle évolutive et la sexualité chez les Basidiomycétes. Ph.D. thesis. University of Paris, Nemours, France
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

