Melanin externalization in Candida albicans depends on cell wall chitin structures
- PMID: 20543065
- PMCID: PMC2937336
- DOI: 10.1128/EC.00051-10
Melanin externalization in Candida albicans depends on cell wall chitin structures
Abstract
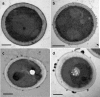
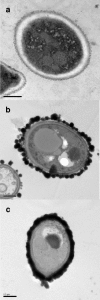
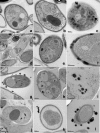
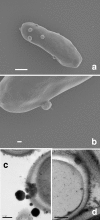
The fungal pathogen Candida albicans produces dark-pigmented melanin after 3 to 4 days of incubation in medium containing l-3,4-dihydroxyphenylalanine (l-DOPA) as a substrate. Expression profiling of C. albicans revealed very few genes significantly up- or downregulated by growth in l-DOPA. We were unable to determine a possible role for melanin in the virulence of C. albicans. However, we showed that melanin was externalized from the fungal cells in the form of electron-dense melanosomes that were free or often loosely bound to the cell wall exterior. Melanin production was boosted by the addition of N-acetylglucosamine to the medium, indicating a possible association between melanin production and chitin synthesis. Melanin externalization was blocked in a mutant specifically disrupted in the chitin synthase-encoding gene CHS2. Melanosomes remained within the outermost cell wall layers in chs3Delta and chs2Delta chs3Delta mutants but were fully externalized in chs8Delta and chs2Delta chs8Delta mutants. All the CHS mutants synthesized dark pigment at equivalent rates from mixed membrane fractions in vitro, suggesting it was the form of chitin structure produced by the enzymes, not the enzymes themselves, that was involved in the melanin externalization process. Mutants with single and double disruptions of the chitinase genes CHT2 and CHT3 and the chitin pathway regulator ECM33 also showed impaired melanin externalization. We hypothesize that the chitin product of Chs3 forms a scaffold essential for normal externalization of melanosomes, while the Chs8 chitin product, probably produced in cell walls in greater quantity in the absence of CHS2, impedes externalization.
Figures





References
-
- Alviano C. S., Farbiarwza S. O., De Souza W., Angluster J., Travassos L. R. 1991. Characterization of Fonsecaea pedrosoi melanin. J. Gen. Microbiol. 137:837–844 - PubMed
-
- Alviano D. S., Franzen A. J., Travassos L. R., Holandino C., Rozental S., Ejzemberg R., Alviano C. S., Rodrigues M. L. 2004. Melanin from Fonsecaea pedrosoi induces production of human antifungal antibodies and enhances the antimicrobial efficacy of phagocytes. Infect. Immun. 72:229–237 - PMC - PubMed
-
- Bell A. A., Wheeler M. H. 1986. Biosynthesis and functions of fungal melanins. Annu. Rev. Phytopathol. 24:411–451
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

