Allergen-induced airway remodeling is impaired in galectin-3-deficient mice
- PMID: 20543100
- PMCID: PMC2918241
- DOI: 10.4049/jimmunol.1000039
Allergen-induced airway remodeling is impaired in galectin-3-deficient mice
Abstract
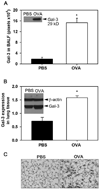
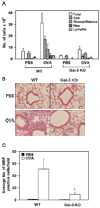
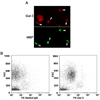
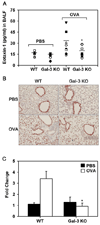
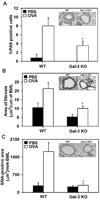
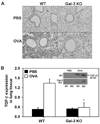
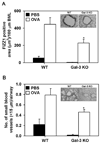
The role played by the beta-galactoside-binding lectin galectin-3 (Gal-3) in airway remodeling, a characteristic feature of asthma that leads to airway dysfunction and poor clinical outcome in humans, was investigated in a murine model of chronic allergic airway inflammation. Wild-type (WT) and Gal-3 knockout (KO) mice were subjected to repetitive allergen challenge with OVA up to 12 wk, and bronchoalveolar lavage fluid (BALF) and lung tissue collected after the last challenge were evaluated for cellular features associated with airway remodeling. Compared to WT mice, chronic OVA challenge in Gal-3 KO mice resulted in diminished remodeling of the airways with significantly reduced mucus secretion, subepithelial fibrosis, smooth muscle thickness, and peribronchial angiogenesis. The higher degree of airway remodeling in WT mice was associated with higher Gal-3 expression in the BALF as well as lung tissue. Cell counts in BALF and lung immunohistology demonstrated that eosinophil infiltration in OVA-challenged Gal-3 KO mice was significantly reduced compared with that WT mice. Evaluation of cellular mediators associated with eosinophil recruitment and airway remodeling revealed that levels of eotaxin-1, IL-5, IL-13, found in inflammatory zone 1, and TGF-beta were substantially lower in Gal-3 KO mice. Finally, leukocytes from Gal-3 KO mice demonstrated decreased trafficking (rolling) on vascular endothelial adhesion molecules compared with that of WT cells. Overall, these studies demonstrate that Gal-3 is an important lectin that promotes airway remodeling via airway recruitment of inflammatory cells, specifically eosinophils, and the development of a Th2 phenotype as well as increased expression of eosinophil-specific chemokines and profibrogenic and angiogenic mediators.
Figures


Similar articles
-
PI3K gamma-deficient mice have reduced levels of allergen-induced eosinophilic inflammation and airway remodeling.Am J Physiol Lung Cell Mol Physiol. 2009 Feb;296(2):L210-9. doi: 10.1152/ajplung.90275.2008. Epub 2008 Nov 21. Am J Physiol Lung Cell Mol Physiol. 2009. PMID: 19028980 Free PMC article.
-
Chronic OVA allergen challenged Siglec-F deficient mice have increased mucus, remodeling, and epithelial Siglec-F ligands which are up-regulated by IL-4 and IL-13.Respir Res. 2010 Nov 1;11(1):154. doi: 10.1186/1465-9921-11-154. Respir Res. 2010. PMID: 21040544 Free PMC article.
-
Endothelial and leukocyte heparan sulfates regulate the development of allergen-induced airway remodeling in a mouse model.Glycobiology. 2014 Aug;24(8):715-27. doi: 10.1093/glycob/cwu035. Epub 2014 May 2. Glycobiology. 2014. PMID: 24794009 Free PMC article.
-
Cytokines and growth factors in airway remodeling in asthma.Curr Opin Immunol. 2007 Dec;19(6):676-80. doi: 10.1016/j.coi.2007.07.017. Epub 2007 Aug 27. Curr Opin Immunol. 2007. PMID: 17720466 Review.
-
Immunologic and inflammatory mechanisms that drive asthma progression to remodeling.J Allergy Clin Immunol. 2008 Mar;121(3):560-70; quiz 571-2. doi: 10.1016/j.jaci.2008.01.031. J Allergy Clin Immunol. 2008. PMID: 18328887 Free PMC article. Review.
Cited by
-
FIZZ1 could enhance the angiogenic ability of rat aortic endothelial cells.Int J Clin Exp Pathol. 2013 Aug 15;6(9):1847-53. eCollection 2013. Int J Clin Exp Pathol. 2013. PMID: 24040449 Free PMC article.
-
Vitamin D Supplementation Reduces Induction of Epithelial-Mesenchymal Transition in Allergen Sensitized and Challenged Mice.PLoS One. 2016 Feb 12;11(2):e0149180. doi: 10.1371/journal.pone.0149180. eCollection 2016. PLoS One. 2016. PMID: 26872336 Free PMC article.
-
Alterations in the proteome of the respiratory tract in response to single and multiple exposures to naphthalene.Proteomics. 2015 Aug;15(15):2655-68. doi: 10.1002/pmic.201400445. Epub 2015 May 13. Proteomics. 2015. PMID: 25825134 Free PMC article.
-
Eosinophil-expressed galectin-3 regulates cell trafficking and migration.Front Pharmacol. 2013 Apr 5;4:37. doi: 10.3389/fphar.2013.00037. eCollection 2013. Front Pharmacol. 2013. PMID: 23576987 Free PMC article.
-
The p110δ subunit of PI3K regulates bone marrow-derived eosinophil trafficking and airway eosinophilia in allergen-challenged mice.Am J Physiol Lung Cell Mol Physiol. 2012 Jun 1;302(11):L1179-91. doi: 10.1152/ajplung.00005.2012. Epub 2012 Mar 16. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22427531 Free PMC article.
References
-
- Bergeron C, Al-Ramli W, Hamid Q. Remodeling in asthma. Proc Am Thorac Soc. 2009;6:301–305. - PubMed
-
- Tagaya E, Tamaoki J. Mechanisms of airway remodeling in asthma. Allergol Int. 2007;56:331–340. - PubMed
-
- Mehrotra AK, Henderson WR., Jr The role of leukotrienes in airway remodeling. Curr Mol Med. 2009;9:383–391. - PubMed
-
- Doherty T, Broide D. Cytokines and growth factors in airway remodeling in asthma. Current Opinion in Immunology. 2007;19:676–680. - PubMed
-
- Sato S, Hughes RC. Regulation of secretion and surface expression of Mac-2, a galactoside-binding protein of macrophages. J Biol Chem. 1994;269:4424–4430. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

