The relative timing of exposure to phagocytosable particulates and to osteoclastogenic cytokines is critically important in the determination of myeloid cell fate
- PMID: 20543106
- PMCID: PMC3016856
- DOI: 10.4049/jimmunol.0902808
The relative timing of exposure to phagocytosable particulates and to osteoclastogenic cytokines is critically important in the determination of myeloid cell fate
Abstract
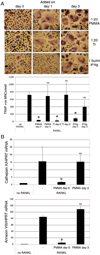
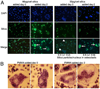
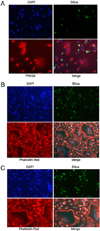
During granulomatous inflammatory reactions, myeloid cells can differentiate into activated phagocytic macrophages, wound-healing macrophages, foreign body giant cells, and bone-resorbing osteoclasts. Although it is appreciated that a variety of stimuli, including cytokines, cell-matrix interactions, and challenge with foreign materials can influence myeloid cell fate, little is known of how these signals integrate during this process. In this study, we have investigated the cross talk between receptor activator of NF-kappaB ligand (RANKL)-induced osteoclastogenesis and particle phagocytosis-induced activation of human monocytes. Understanding interconnected signals is of particular importance to disorders, such as periprosthetic osteolysis, in which granulomatous inflammation is initiated by particle phagocytosis in proximity to bone and leads to inflammatory bone loss. Using cell-based osteoclastogenesis and phagocytosis assays together with expression analysis of key regulators of osteoclastogenesis, we show in this study that phagocytosis of disease-relevant particles inhibits RANKL-mediated osteoclastogenesis of human monocytes. Mechanistically, phagocytosis mediates this effect by downregulation of RANK and c-Fms, the receptors for the essential osteoclastogenic cytokines RANKL and M-CSF. RANKL pretreatment of monocytes generates preosteoclasts that are resistant to RANK downregulation and committed to osteoclast formation, even though they retain phagocytic activity. Thus, the relative timing of exposure to phagocytosable particulates and to osteoclastogenic cytokines is critically important in the determination of myeloid cell fate.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures




Similar articles
-
Receptor activator of NF-kappaB ligand induces the expression of carbonic anhydrase II, cathepsin K, and matrix metalloproteinase-9 in osteoclast precursor RAW264.7 cells.Life Sci. 2007 Mar 13;80(14):1311-8. doi: 10.1016/j.lfs.2006.12.037. Epub 2007 Jan 23. Life Sci. 2007. PMID: 17306833
-
BSP and RANKL induce osteoclastogenesis and bone resorption synergistically.J Bone Miner Res. 2005 Sep;20(9):1669-79. doi: 10.1359/JBMR.050511. Epub 2005 May 16. J Bone Miner Res. 2005. PMID: 16059638
-
NMDA glutamate receptors are expressed by osteoclast precursors and involved in the regulation of osteoclastogenesis.J Cell Biochem. 2003 Oct 1;90(2):424-36. doi: 10.1002/jcb.10625. J Cell Biochem. 2003. PMID: 14505357
-
Interleukin-6 inhibits receptor activator of nuclear factor kappaB ligand-induced osteoclastogenesis by diverting cells into the macrophage lineage: key role of Serine727 phosphorylation of signal transducer and activator of transcription 3.Endocrinology. 2008 Jul;149(7):3688-97. doi: 10.1210/en.2007-1719. Epub 2008 Apr 10. Endocrinology. 2008. PMID: 18403479
-
The osteoclastogenic molecules RANKL and RANK are associated with periprosthetic osteolysis.J Bone Joint Surg Br. 2001 Aug;83(6):902-11. doi: 10.1302/0301-620x.83b6.10905. J Bone Joint Surg Br. 2001. PMID: 11521937 Review.
Cited by
-
Rho GTPase expression in human myeloid cells.PLoS One. 2012;7(8):e42563. doi: 10.1371/journal.pone.0042563. Epub 2012 Aug 16. PLoS One. 2012. PMID: 22916134 Free PMC article.
-
Genome-Wide Association Identifies Risk Pathways for SAPHO Syndrome.Front Cell Dev Biol. 2021 Mar 18;9:643644. doi: 10.3389/fcell.2021.643644. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33816493 Free PMC article.
-
Resistance to anticancer vaccination effect is controlled by a cancer cell-autonomous phenotype that disrupts immunogenic phagocytic removal.Oncotarget. 2015 Sep 29;6(29):26841-60. doi: 10.18632/oncotarget.4754. Oncotarget. 2015. PMID: 26314964 Free PMC article.
-
Targeting the giant cell tumor stromal cell: functional characterization and a novel therapeutic strategy.PLoS One. 2013 Jul 26;8(7):e69101. doi: 10.1371/journal.pone.0069101. Print 2013. PLoS One. 2013. PMID: 23922683 Free PMC article.
-
Feedback inhibition of osteoclastogenesis during inflammation by IL-10, M-CSF receptor shedding, and induction of IRF8.Ann N Y Acad Sci. 2011 Nov;1237:88-94. doi: 10.1111/j.1749-6632.2011.06217.x. Ann N Y Acad Sci. 2011. PMID: 22082370 Free PMC article. Review.
References
-
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. - PubMed
-
- Ross FP. M-CSF, c-Fms, and signaling in osteoclasts and their precursors. Ann. N. Y. Acad. Sci. 2006;1068:110–116. - PubMed
-
- Ha H, Lee JH, Kim HN, Kwak HB, Kim HM, Lee SE, Rhee JH, Kim HH, Lee ZH. Stimulation by TLR5 modulates osteoclast differentiation through STAT1/IFN-beta. J. Immunol. 2008;180:1382–1389. - PubMed
-
- Takami M, Kim N, Rho J, Choi Y. Stimulation by toll-like receptors inhibits osteoclast differentiation. J. Immunol. 2002;169:1516–1523. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

