The adaptor molecule Act1 regulates BAFF responsiveness and self-reactive B cell selection during transitional B cell maturation
- PMID: 20543113
- PMCID: PMC3225255
- DOI: 10.4049/jimmunol.0903312
The adaptor molecule Act1 regulates BAFF responsiveness and self-reactive B cell selection during transitional B cell maturation
Abstract
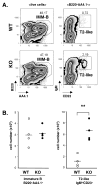
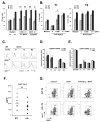
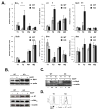
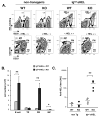
The transitional stage is a key check-point for elimination of autoreactive B cells in the periphery. This selection process requires fine regulation of signals received through BCR and B cell activating factor (BAFF) receptor. We previously identified the adaptor molecule Act1 as a negative regulator of BAFF-mediated signaling. Deficiency of Act1 in mice results in peripheral B cell hyperplasia and development of autoimmunity. In this study, we demonstrate that Act1 plays a critical role in the regulation of transitional B cell survival and maturation. We found that the ratio of late-transitional (T2) to early-transitional (T1) cells was increased in spleens from Act1-deficient mice. Moreover, BAFF stimulation induced better T1 cell survival and promoted more efficient maturation of T1 cells into T2 cells ex vivo in the absence of Act1. BAFF stimulation induced higher levels of the anti-apoptotic Bcl-2 member Mc1-l in Act1-deficient T1 cells than in wild-type control cells, suggesting that Mcl-1 might be one of the key effector molecules for BAFF-mediated survival of the Act1-deficient transitional B cells. Importantly, costimulation with BAFF was able to rescue Act1-deficient T1 cells from BCR-induced apoptosis more effectively than Act1-sufficient T1 B cells. Finally, by using hen egg lysozyme double transgenic mice, we demonstrated that Act1 deficiency can promote the maturation of Ag-specific autoreactive B cells. Taken together, our results suggest that the transitional stage is a critical point of action of Act1 in the elimination of autoreactive B cells and in the regulation of peripheral B cell homeostasis.
Conflict of interest statement
The authors declare no financial or commercial conflicts of interests.
Figures


Similar articles
-
Absence of mature peripheral B cell populations in mice with concomitant defects in B cell receptor and BAFF-R signaling.J Immunol. 2009 Nov 1;183(9):5630-43. doi: 10.4049/jimmunol.0901100. J Immunol. 2009. PMID: 19843948
-
Deficiency of Act1, a critical modulator of B cell function, leads to development of Sjögren's syndrome.Eur J Immunol. 2008 Aug;38(8):2219-28. doi: 10.1002/eji.200738113. Eur J Immunol. 2008. PMID: 18624351 Free PMC article.
-
TLR4 promotes B cell maturation: independence and cooperation with B lymphocyte-activating factor.J Immunol. 2010 May 1;184(9):4662-72. doi: 10.4049/jimmunol.0903253. Epub 2010 Mar 31. J Immunol. 2010. PMID: 20357250
-
Act1 modulates autoimmunity through its dual functions in CD40L/BAFF and IL-17 signaling.Cytokine. 2008 Feb;41(2):105-13. doi: 10.1016/j.cyto.2007.09.015. Epub 2007 Dec 3. Cytokine. 2008. PMID: 18061473 Review.
-
Regulation of B cell self-tolerance by BAFF.Semin Immunol. 2006 Oct;18(5):276-83. doi: 10.1016/j.smim.2006.04.003. Epub 2006 Aug 17. Semin Immunol. 2006. PMID: 16916609 Review.
Cited by
-
Differential Roles of IDO1 and IDO2 in T and B Cell Inflammatory Immune Responses.Front Immunol. 2020 Aug 18;11:1861. doi: 10.3389/fimmu.2020.01861. eCollection 2020. Front Immunol. 2020. PMID: 32973768 Free PMC article.
-
Iguratimod represses B cell terminal differentiation linked with the inhibition of PKC/EGR1 axis.Arthritis Res Ther. 2019 Apr 11;21(1):92. doi: 10.1186/s13075-019-1874-2. Arthritis Res Ther. 2019. PMID: 30971291 Free PMC article.
-
Regulatory effect of Act1 on the BAFF pathway in B-cell malignancy.Oncol Lett. 2019 Apr;17(4):3727-3734. doi: 10.3892/ol.2019.10047. Epub 2019 Feb 18. Oncol Lett. 2019. PMID: 30930983 Free PMC article.
-
Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease.Immunol Rev. 2011 Nov;244(1):115-33. doi: 10.1111/j.1600-065X.2011.01067.x. Immunol Rev. 2011. PMID: 22017435 Free PMC article. Review.
-
The cytokine CSBF inhibits the IL-17A and TNF-α inflammatory pathways via SUSD2-ACT1 in keratinocytes and alleviates IMQ-induced psoriasis.Cell Mol Immunol. 2025 Aug 14. doi: 10.1038/s41423-025-01325-3. Online ahead of print. Cell Mol Immunol. 2025. PMID: 40804449
References
-
- Basten A, Brink R, Peake P, Adams E, Crosbie J, Hartley S, Goodnow CC. Self Tolerance in the B-Cell Repertoire. Immunological Reviews. 1991;122:5–19. - PubMed
-
- Shlomchik MJ. Sites and stages of autoreactive B cell activation and regulation. Immunity. 2008;28:18–28. - PubMed
-
- Hardy RR, Li YS, Allman D, Asano M, Gui M, Hayakawa K. B-cell commitment, development and selection. Immunological Reviews. 2000;175:23–32. - PubMed
-
- Monroe JG, Bannish G, Fuentes-Panana EM, King LB, Sandel PC, Chung J, Sater R. Positive and negative selection during B lymphocyte development. Immunologic Research. 2003;27:427–442. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

