Shear stress and the endothelial transport barrier
- PMID: 20543206
- PMCID: PMC2915475
- DOI: 10.1093/cvr/cvq146
Shear stress and the endothelial transport barrier
Abstract
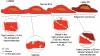
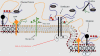
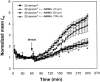
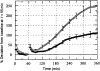
The shear stress of flowing blood on the surfaces of endothelial cells that provide the barrier to transport of solutes and water between blood and the underlying tissue modulates the permeability to solutes and the hydraulic conductivity. This review begins with a discussion of transport pathways across the endothelium and then considers the experimental evidence from both in vivo and in vitro studies that shows an influence of shear stress on endothelial transport properties after both acute (minutes to hours) and chronic (hours to days) changes in shear stress. Next, the effects of shear stress on individual transport pathways (tight junctions, adherens junctions, vesicles and leaky junctions) are described, and this information is integrated with the transport experiments to suggest mechanisms controlling both acute and chronic responses of transport properties to shear stress. The review ends with a summary of future research challenges.
Figures

Similar articles
-
Glycocalyx modulates the motility and proliferative response of vascular endothelium to fluid shear stress.Am J Physiol Heart Circ Physiol. 2007 Aug;293(2):H1023-30. doi: 10.1152/ajpheart.00162.2007. Epub 2007 Apr 27. Am J Physiol Heart Circ Physiol. 2007. PMID: 17468337
-
Effect of endothelial glycocalyx on water and LDL transport through the rat abdominal aorta.Am J Physiol Heart Circ Physiol. 2021 Apr 1;320(4):H1724-H1737. doi: 10.1152/ajpheart.00861.2020. Epub 2021 Mar 12. Am J Physiol Heart Circ Physiol. 2021. PMID: 33710913
-
Visualization of three pathways for macromolecule transport across cultured endothelium and their modification by flow.Am J Physiol Heart Circ Physiol. 2017 Nov 1;313(5):H959-H973. doi: 10.1152/ajpheart.00218.2017. Epub 2017 Jul 28. Am J Physiol Heart Circ Physiol. 2017. PMID: 28754719 Free PMC article.
-
The endothelial glycocalyx: a mechano-sensor and -transducer.Sci Signal. 2008 Oct 7;1(40):pt8. doi: 10.1126/scisignal.140pt8. Sci Signal. 2008. PMID: 18840877 Review.
-
Vascular permeability modulation at the cell, microvessel, or whole organ level: towards closing gaps in our knowledge.Cardiovasc Res. 2010 Jul 15;87(2):218-29. doi: 10.1093/cvr/cvq115. Epub 2010 Apr 23. Cardiovasc Res. 2010. PMID: 20418473 Free PMC article. Review.
Cited by
-
Three-dimensional microfluidic collagen hydrogels for investigating flow-mediated tumor-endothelial signaling and vascular organization.Tissue Eng Part C Methods. 2014 Jan;20(1):64-75. doi: 10.1089/ten.TEC.2012.0731. Epub 2013 Jul 12. Tissue Eng Part C Methods. 2014. PMID: 23730946 Free PMC article.
-
Biomimetic Oil-in-Water Nanoemulsions as a Suitable Drug Delivery System to Target Inflamed Endothelial Cells.Nanomaterials (Basel). 2024 Jul 31;14(15):1286. doi: 10.3390/nano14151286. Nanomaterials (Basel). 2024. PMID: 39120393 Free PMC article.
-
Tonic regulation of vascular permeability.Acta Physiol (Oxf). 2013 Apr;207(4):628-49. doi: 10.1111/apha.12076. Epub 2013 Feb 25. Acta Physiol (Oxf). 2013. PMID: 23374222 Free PMC article. Review.
-
Permeability of Epithelial/Endothelial Barriers in Transwells and Microfluidic Bilayer Devices.Micromachines (Basel). 2019 Aug 13;10(8):533. doi: 10.3390/mi10080533. Micromachines (Basel). 2019. PMID: 31412604 Free PMC article.
-
Recapitulating essential pathophysiological characteristics in lung-on-a-chip for disease studies.Front Immunol. 2023 Feb 28;14:1093460. doi: 10.3389/fimmu.2023.1093460. eCollection 2023. Front Immunol. 2023. PMID: 36926347 Free PMC article. Review.
References
-
- Tarbell JM. Mass transport in arteries and the localization of atherosclerosis. Annu Rev Biomed Eng. 2003;5:79–118. doi:10.1146/annurev.bioeng.5.040202.121529. - DOI - PubMed
-
- Simionescu M, Simionescu N. Handbook of Physiology The Cardiovascular System Microcirculation. IV, Chapter 3. Bethesda, MD: American Physiological Society; 1984. Ultrastructure of the microvessel wall: functional correlations; pp. 41–101.
-
- Michel CC. Transport of macromolecules through microvascular walls. Cardiovasc Res. 1996;32:644–653. - PubMed
-
- Ogunrinade O, Kameya GT, Truskey GA. Effect of fluid shear stress on the permeability of the arterial endothelium. Ann Biomed Eng. 2002;30:430–446. doi:10.1114/1.1467924. - DOI - PubMed
-
- Fu BM, Shen S. Acute VEGF effect on solute permeability of mammalian microvessels in vivo. Microvasc Res. 2004;68:51–62. doi:10.1016/j.mvr.2004.03.004. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

