Na+ imaging reveals little difference in action potential-evoked Na+ influx between axon and soma
- PMID: 20543843
- PMCID: PMC3102307
- DOI: 10.1038/nn.2574
Na+ imaging reveals little difference in action potential-evoked Na+ influx between axon and soma
Abstract
In cortical pyramidal neurons, the axon initial segment (AIS) is pivotal in synaptic integration. It has been asserted that this is because there is a high density of Na(+) channels in the AIS. However, we found that action potential-associated Na(+) flux, as measured by high-speed fluorescence Na(+) imaging, was about threefold larger in the rat AIS than in the soma. Spike-evoked Na(+) flux in the AIS and the first node of Ranvier was similar and was eightfold lower in basal dendrites. At near-threshold voltages, persistent Na(+) conductance was almost entirely axonal. On a time scale of seconds, passive diffusion, and not pumping, was responsible for maintaining transmembrane Na(+) gradients in thin axons during high-frequency action potential firing. In computer simulations, these data were consistent with the known features of action potential generation in these neurons.
Figures


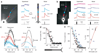
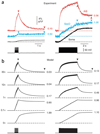
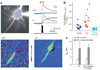
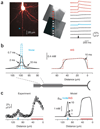

Comment in
-
The Na+ channel conundrum: axon structure versus function.Nat Neurosci. 2010 Jul;13(7):784-5. doi: 10.1038/nn0710-784. Nat Neurosci. 2010. PMID: 20581812 No abstract available.
References
-
- Kole MH, Stuart GJ. Is action potential threshold lowest in the axon? Nat. Neurosci. 2008;11:1253–1255. - PubMed
-
- Colbert CM, Pan E. Ion channel properties underlying axonal action potential initiation in pyramidal neurons. Nat. Neurosci. 2002;5:533–538. - PubMed
-
- Kole MH, et al. Action potential generation requires a high sodium channel density in the axon initial segment. Nat. Neurosci. 2008;11:178–186. - PubMed
-
- Hu W, et al. Distinct contributions of Na(v)1.6 and Na(v)1.2 in action potential initiation and backpropagation. Nat. Neurosci. 2009;12:996–1002. - PubMed
-
- Stuart G, Sakmann B. Amplification of EPSPs by axosomatic sodium channels in neocortical pyramidal neurons. Neuron. 1995;15:1065–1076. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

