Tracking antigen-specific T-cells during clinical tolerance induction in humans
- PMID: 20543955
- PMCID: PMC2882953
- DOI: 10.1371/journal.pone.0011028
Tracking antigen-specific T-cells during clinical tolerance induction in humans
Abstract
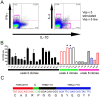
Allergen immunotherapy presents an opportunity to define mechanisms of induction of clinical tolerance in humans. Significant progress has been made in our understanding of changes in T cell responses during immunotherapy, but existing work has largely been based on functional T cell assays. HLA-peptide-tetrameric complexes allow the tracking of antigen-specific T-cell populations based on the presence of specific T-cell receptors and when combined with functional assays allow a closer assessment of the potential roles of T-cell anergy and clonotype evolution. We sought to develop tools to facilitate tracking of antigen-specific T-cell populations during wasp-venom immunotherapy in people with wasp-venom allergy. We first defined dominant immunogenic regions within Ves v 5, a constituent of wasp venom that is known to represent a target antigen for T-cells. We next identified HLA-DRB1*1501 restricted epitopes and used HLA class II tetrameric complexes alongside cytokine responses to Ves v 5 to track T-cell responses during immunotherapy. In contrast to previous reports, we show that there was a significant initial induction of IL-4 producing antigen-specific T-cells within the first 3-5 weeks of immunotherapy which was followed by reduction of circulating effector antigen-specific T-cells despite escalation of wasp-venom dosage. However, there was sustained induction of IL-10-producing and FOXP3 positive antigen-specific T cells. We observed that these IL-10 producing cells could share a common precursor with IL-4-producing T cells specific for the same epitope. Clinical tolerance induction in humans is associated with dynamic changes in frequencies of antigen-specific T-cells, with a marked loss of IL-4-producing T-cells and the acquisition of IL-10-producing and FOXP3-positive antigen-specific CD4+ T-cells that can derive from a common shared precursor to pre-treatment effector T-cells. The development of new approaches to track antigen specific T-cell responses during immunotherapy can provide novel insights into mechanisms of tolerance induction in humans and identify new potential treatment targets.
Conflict of interest statement
Figures

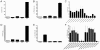
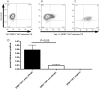
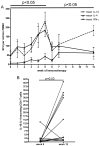


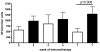
References
-
- Muller U, Thurnheer U, Patrizzi R, Spiess J, Hoigne R. Immunotherapy in bee sting hypersensitivity. Bee venom versus wholebody extract. Allergy. 1979;34:369–378. - PubMed
-
- Gillman SA, Cummins LH, Kozak PP, Jr, Hoffman DR. Venom immunotherapy: comparison of “rush” vs “conventional” schedules. Ann Allergy. 1980;45:351–354. - PubMed
-
- Bousquet J, Muller UR, Dreborg S, Jarisch R, Malling HJ, et al. Immunotherapy with Hymenoptera venoms. Position paper of the Working Group on Immunotherapy of the European Academy of Allergy and Clinical Immunology. Allergy. 1987;42:401–413. - PubMed
-
- Bousquet J, Fontez A, Aznar R, Robinet-Levy M, Michel FB. Combination of passive and active immunization in honeybee venom immunotherapy. J Allergy Clin Immunol. 1987;79:947–954. - PubMed
-
- Nouri-Aria KT, Wachholz PA, Francis JN, Jacobson MR, Walker SM, et al. Grass pollen immunotherapy induces mucosal and peripheral IL-10 responses and blocking IgG activity. J Immunol. 2004;172:3252–3259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

