Cell cycle and anti-estrogen effects synergize to regulate cell proliferation and ER target gene expression
- PMID: 20543978
- PMCID: PMC2882356
- DOI: 10.1371/journal.pone.0011011
Cell cycle and anti-estrogen effects synergize to regulate cell proliferation and ER target gene expression
Abstract
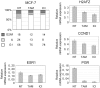
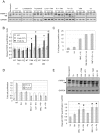
Antiestrogens are designed to antagonize hormone induced proliferation and ERalpha target gene expression in mammary tumor cells. Commonly used drugs such as OH-Tamoxifen and ICI 182780 (Fulvestrant) block cell cycle progression in G0/G1. Inversely, the effect of cell cycle stage on ER regulated gene expression has not been tested directly. We show that in ERalpha-positive breast cancer cells (MCF-7) the estrogen receptor gene and downstream target genes are cell cycle regulated with expression levels varying as much as three-fold between phases of the cell cycle. Steroid free culture conditions commonly used to assess the effect of hormones or antiestrogens on gene expression also block MCF-7 cells in G1-phase when several ERalpha target genes are overexpressed. Thus, cell cycle effects have to be taken into account when analyzing the impact of hormonal treatments on gene transcription. We found that antiestrogens repress transcription of several ERalpha target genes specifically in S phase. This observation corroborates the more rapid and strong impact of antiestrogen treatments on cell proliferation in thymidine, hydroxyurea or aphidicolin arrested cells and correlates with an increase of apoptosis compared to similar treatments in lovastatin or nocodazol treated cells. Hence, cell cycle effects synergize with the action of antiestrogens. An interesting therapeutic perspective could be to enhance the action of anti-estrogens by associating hormone-therapy with specific cell cycle drugs.
Conflict of interest statement
Figures




Similar articles
-
Estrogen receptor alpha is cell cycle-regulated and regulates the cell cycle in a ligand-dependent fashion.Cell Cycle. 2016 Jun 17;15(12):1579-90. doi: 10.1080/15384101.2016.1166327. Epub 2016 Apr 6. Cell Cycle. 2016. PMID: 27049344 Free PMC article.
-
Domains of estrogen receptor alpha (ERalpha) required for ERalpha/Sp1-mediated activation of GC-rich promoters by estrogens and antiestrogens in breast cancer cells.Mol Endocrinol. 2003 May;17(5):804-17. doi: 10.1210/me.2002-0406. Epub 2003 Feb 6. Mol Endocrinol. 2003. PMID: 12576490
-
Activation of the human estrogen receptor by the antiestrogens ICI 182,780 and tamoxifen in yeast genetic systems: implications for their mechanism of action.Proc Natl Acad Sci U S A. 2000 Mar 28;97(7):3696-701. doi: 10.1073/pnas.97.7.3696. Proc Natl Acad Sci U S A. 2000. PMID: 10725345 Free PMC article.
-
Molecular mechanisms of estrogen action: selective ligands and receptor pharmacology.J Steroid Biochem Mol Biol. 2000 Nov 30;74(5):279-85. doi: 10.1016/s0960-0760(00)00104-7. J Steroid Biochem Mol Biol. 2000. PMID: 11162936 Review.
-
Antiestrogens--tamoxifen, SERMs and beyond.Invest New Drugs. 1999;17(3):285-311. doi: 10.1023/a:1006348907994. Invest New Drugs. 1999. PMID: 10665480 Review.
Cited by
-
The diaryl-imidazopyridazine anti-plasmodial compound, MMV652103, exhibits anti-breast cancer activity.EXCLI J. 2022 Apr 4;21:656-679. doi: 10.17179/excli2021-4323. eCollection 2022. EXCLI J. 2022. PMID: 35651652 Free PMC article.
-
Endocrine resistance in breast cancer--An overview and update.Mol Cell Endocrinol. 2015 Dec 15;418 Pt 3(0 3):220-34. doi: 10.1016/j.mce.2015.09.035. Epub 2015 Oct 9. Mol Cell Endocrinol. 2015. PMID: 26455641 Free PMC article. Review.
-
Enhanced anti-mammary gland cancer activities of tamoxifen-loaded erythropoietin-coated drug delivery system.PLoS One. 2019 Jul 10;14(7):e0219285. doi: 10.1371/journal.pone.0219285. eCollection 2019. PLoS One. 2019. PMID: 31291309 Free PMC article.
-
HR+, HER2- Advanced Breast Cancer and CDK4/6 Inhibitors: Mode of Action, Clinical Activity, and Safety Profiles.Curr Cancer Drug Targets. 2017;17(7):637-649. doi: 10.2174/1568009617666170330120452. Curr Cancer Drug Targets. 2017. PMID: 28359238 Free PMC article. Review.
-
ER-/ER+ breast cancer cell lines exhibited different resistance to paclitaxel through pulse selection.Med Oncol. 2012 Jun;29(2):495-502. doi: 10.1007/s12032-011-9889-9. Epub 2011 Mar 12. Med Oncol. 2012. PMID: 21399998
References
-
- Henderson BE, Ross R, Bernstein L. Estrogens as a cause of human cancer: the Richard and Hinda Rosenthal Foundation award lecture. Cancer Res. 1988;48:246–253. - PubMed
-
- Prall OW, Rogan EM, Sutherland RL. Estrogen regulation of cell cycle progression in breast cancer cells. J Steroid Biochem Mol Biol. 1998;65:169–174. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

