Rapamycin pharmacokinetic and pharmacodynamic relationships in osteosarcoma: a comparative oncology study in dogs
- PMID: 20543980
- PMCID: PMC2882366
- DOI: 10.1371/journal.pone.0011013
Rapamycin pharmacokinetic and pharmacodynamic relationships in osteosarcoma: a comparative oncology study in dogs
Abstract
Background: Signaling through the mTOR pathway contributes to growth, progression and chemoresistance of several cancers. Accordingly, inhibitors have been developed as potentially valuable therapeutics. Their optimal development requires consideration of dose, regimen, biomarkers and a rationale for their use in combination with other agents. Using the infrastructure of the Comparative Oncology Trials Consortium many of these complex questions were asked within a relevant population of dogs with osteosarcoma to inform the development of mTOR inhibitors for future use in pediatric osteosarcoma patients.
Methodology/principal findings: This prospective dose escalation study of a parenteral formulation of rapamycin sought to define a safe, pharmacokinetically relevant, and pharmacodynamically active dose of rapamycin in dogs with appendicular osteosarcoma. Dogs entered into dose cohorts consisting of 3 dogs/cohort. Dogs underwent a pre-treatment tumor biopsy and collection of baseline PBMC. Dogs received a single intramuscular dose of rapamycin and underwent 48-hour whole blood pharmacokinetic sampling. Additionally, daily intramuscular doses of rapamycin were administered for 7 days with blood rapamycin trough levels collected on Day 8, 9 and 15. At Day 8 post-treatment collection of tumor and PBMC were obtained. No maximally tolerated dose of rapamycin was attained through escalation to the maximal planned dose of 0.08 mg/kg (2.5 mg/30 kg dog). Pharmacokinetic analysis revealed a dose-dependent exposure. In all cohorts modulation of the mTOR pathway in tumor and PBMC (pS6RP/S6RP) was demonstrated. No change in pAKT/AKT was seen in tumor samples following rapamycin therapy.
Conclusions/significance: Rapamycin may be safely administered to dogs and can yield therapeutic exposures. Modulation pS6RP/S6RP in tumor tissue and PBMCs was not dependent on dose. Results from this study confirm that the dog may be included in the translational development of rapamycin and potentially other mTOR inhibitors. Ongoing studies of rapamycin in dogs will define optimal schedules for their use in cancer and evaluate the role of rapamycin use in the setting of minimal residual disease.
Conflict of interest statement
Figures



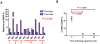

References
-
- Hutson TE, Figlin RA. Renal cell cancer. Cancer J. 2007;13:282–286. - PubMed
-
- Wan X, Helman LJ. The biology behind mTOR inhibition in sarcoma. Oncologist. 2007;12:1007–1018. - PubMed
-
- Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. - PubMed
-
- Yao JC. Neuroendocrine tumors. Molecular targeted therapy for carcinoid and islet-cell carcinoma. Best Pract Res Clin Endocrinol Metab. 2007;21:163–172. - PubMed
-
- Costa LJ. Aspects of mTOR biology and the use of mTOR inhibitors in non-Hodgkin's lymphoma. Cancer Treat Rev. 2007;33:78–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

